Synthesis of ferrocenyl hyper-branched polyethylene for non-covalent dispersion of multi-walled carbon nanotubes and fabrication of flexible carbon nanotubes-based conductive films†
Zheng Deng,
Li Wang*,
Haojie Yu*,
Xiaoting Zhai,
Yongsheng Chen,
Zain-ul-Abdin and
Nasir M. Abbasi
State Key Laboratory of Chemical Engineering, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: dz0909@qq.com; opl_wl@dial.zju.edu.cn; hjyu@zju.edu.cn; 1052204961@qq.com; josephchan0314@gmail.com; zainulabdinchm@gmail.com; abbasichemist786@gmail.com; Fax: +86 0571 8795 1612; Tel: +86 0571 8795 3200
First published on 11th March 2016
Abstract
Ferrocenyl hyper-branched polyethylene (HBPE-g-PFcEMA) was synthesized by chain-walking polymerization followed by atom transfer radical polymerization, and then characterized by 1H NMR and GPC. HBPE-g-PFcEMA was subsequently employed to disperse multi-walled carbon nanotubes (MWCNTs) in CHCl3. It was found that the MWCNTs were efficiently dispersed by HBPE-g-PFcEMA at a concentration of 150.4 mg L−1. The dispersibility of the MWCNTs in the presence of HBPE-g-PFcEMA was evaluated by TEM and SEM. The π–π stacking interactions between the MWCNTs and HBPE-g-PFcEMA were confirmed by UV-vis spectroscopy. Fabrication of the flexible conductive film by spin-coating the MWCNTs/HBPE-g-PFcEMA dispersion on a PET substrate was explored. The resultant conductive film showed good conductivity (conductivity = 943.4 S cm−1). Furthermore, its good conductivity was retained even after being twisted or bent. Thus, the conductive film might find its potential application in flexible electronics.
Conductive films have been the subject of an emerging and rapidly progressing research area, especially, for use in organic light-emitting diodes,1 solar cells2 and touch panels.3 Indium tin oxide (ITO) has been the most commonly used conductive material in the past few decades, mostly due to its high transparency. However, its brittle ceramic nature limits its further progress. Carbon nanotubes (CNTs), which have excellent thermal, mechanical and electrical properties, are considered as one of the most important alternatives to ITO.4–6 However, CNTs have low solubility in solvents and they tend to aggregate together, which greatly hinder their wider application. To tackle this problem, both covalent and non-covalent methods have been employed.7 Between these two methods, the non-covalent method is preferred, mainly because it preserves the electronic network of the nanotubes.8
Non-covalent dispersion of the nanotubes employing polymers has attracted significant interest, as it offers the advantages of fabricating CNTs/polymer composite conductive films.9,10 This method is mainly based on π–π stacking and other physical interactions between the CNTs and polymers.11,12 Thus, the key point for designing a new polymer-based dispersant is that the polymer must contain functional groups that can interact with the CNTs. Usually, the conjugated side group (e.g. pyrene,13 porphyrin,14 benzene,15,16 etc.) or conjugated backbone,17 which have π–π stacking interactions with CNTs, is employed in the construction of the polymer-based dispersants. Besides the interactions between the CNTs and polymers, the structure of the polymers should also be taken into consideration when designing new polymer-based dispersants. In comparison with the linear polymers, hyper-branched polymers are considered to be a better choice, due to their highly branched structure and abundant terminal groups.18 Furthermore, their lower melting point, lower solution viscosity and higher solubility will favour the fabrication of CNTs/polymer composite films. Although various polymers have been applied to disperse CNTs successfully, the exploration of new polymer-based dispersants that are able to disperse CNTs with high concentration is still a challenging research topic at the present time.
Recently, hyper-branched polyethylene (HBPE), with its highly branched architecture (about 100 branches per 1000 carbons), was found to have the ability to disperse CNTs in organic solvents at high concentration, due to the presence of abundant branch ends in HBPE, which contributes to the high density and thus to the CH–π interactions between HBPE and MWCNTs.19,20 However, HBPE is an amorphous material with a melting endotherm at about −33 °C, due to its unique hyper-branched structure.21 Thus, the CNTs/HBPE composite film is hardly utilized in practical devices. Moreover, the choices of polymers to prepare composites with HBPE are limited due to its relative low compatibility.22,23 Hence, the synthesis of HBPE-based polymers that have the ability to disperse CNTs efficiently and feasibly for the fabrication of a conductive film is of significant interest. Various monomers24 have been utilized to synthesize HBPE-based polymers. Considering that CH–π interactions are much weaker than the π–π stacking interactions, monomers that have π–π stacking interactions with CNTs are preferred. Ferrocene-containing monomers, consisting of two cyclopentadienyl rings bound on opposite sides of a central iron atom, have been recently found to have π–π stacking interactions with CNTs, and consequently several ferrocene-containing polymers have been employed to disperse CNTs in organic solvents efficiently.25–27 Moreover, ferrocene-containing polymers have been found to have good film-forming properties.28–30
Herein, a ferrocene-containing polymer was grafted from the HBPE core to provide an efficient hyper-branched-polymer-based dispersant (HBPE-g-PFcEMA) for the non-covalent dispersion of MWCNTs, and which had the ability to disperse CNTs and could be used to fabricate conductive films. The inner HBPE endowed the ferrocenyl HBPE with a hyper-branched structure, which provided the de-bundled CNTs with solubility and stability. The outer ferrocene-containing blocks acted as anchors to wrap the CNTs through π–π stacking interactions. Also, the ferrocene-containing blocks endowed the HBPE-g-PFcEMA with good film-forming properties. Furthermore, the cost of ferrocenyl hyper-branched polyethylene should be comparable to the conventional non-covalent dispersant for CNTs since ethylene and ferrocene are commercially available and are cheap raw materials.
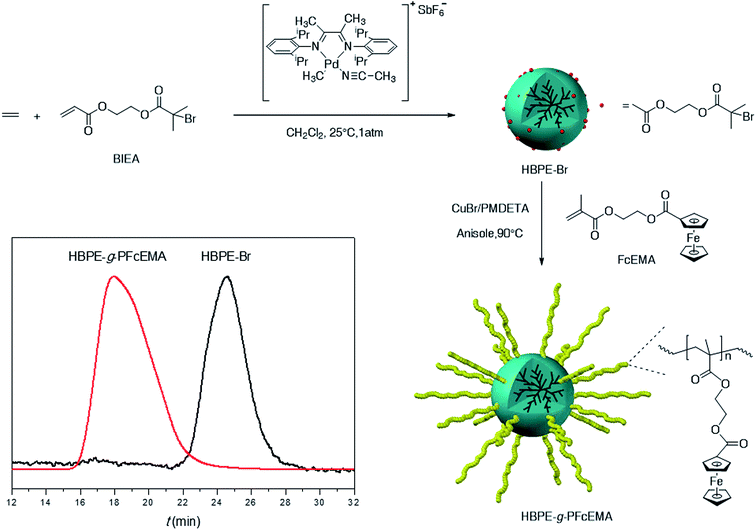
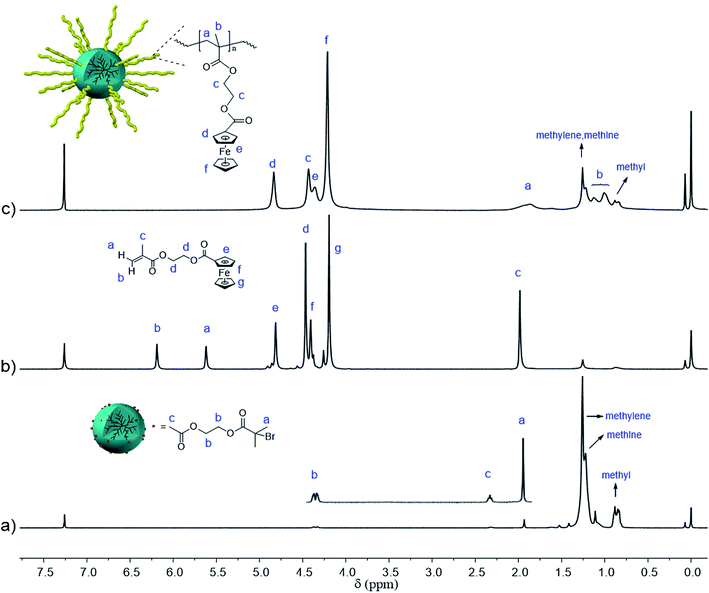
To synthesize HBPE-g-PFcEMA, a hyper-branched polyethylene macro-initiator, namely HBPE-Br, was synthesized by chain-walking polymerization (CWP), which was subsequently utilized to initiate the atom transfer radical polymerization (ATRP) of 2-(methacryloyloxy)-ethyl ferrocenecarboxylate (FcEMA) (Scheme 1) according to the literature.31 The chemical structure of HBPE-g-PFcEMA was characterized by 1H NMR spectroscopy. As shown in Fig. 1, obvious characteristic resonances of the PFcEMA segment at 4.83 ppm (s, 2H, Hd), 4.43 ppm (s, 4H, Hc), 4.36 ppm (s, 2H, He) and 4.21 ppm can be observed in Fig. 1c, which indicate the successful grafting of the PFcEMA segments onto the HBPE. The number average molecular weight (Mn = 1190 kg mol−1) and polydispersity of HBPE-g-PFcEMA (PDI = 2.75) were obtained from the GPC results using a LS detector (Scheme 1).
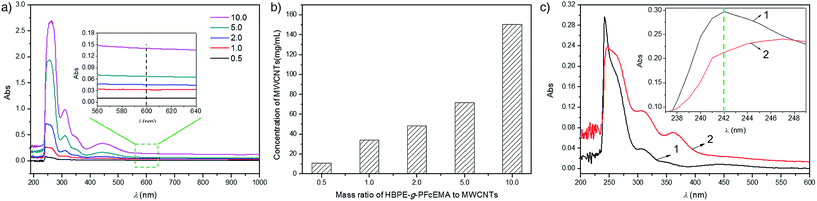
The HBPE-g-PFcEMA was employed to disperse MWCNTs in CHCl3 under ultrasonication at 20 °C. The concentration of the MWCNTs in CHCl3 was determined by UV-vis spectroscopy (Fig. 2a) using a specific extinction coefficient (0.093 L cm−1 mg−1), which was obtained using hyper-branched polyethylene (Fig. S1a–c†) according to the literature.19 The concentration of MWCNTs at different mass ratio of HBPE-g-PFcEMA/MWCNTs was summarized at Fig. 2b. It was found that the concentration of the MWCNTs increased along with the increase in the mass ratio of HBPE-g-PFcEMA/MWCNTs. This was mainly because more HBPE-g-PFcEMA was attached to the surface of the MWCNTs at higher mass ratios of HBPE-g-PFcEMA/MWCNTs, which prevented aggregation of the MWCNTs.21,25,26 The maximum concentration (150.4 mg L−1) was achieved at a mass ratio of 10.0, which was comparable to the previously reported values obtained by the non-covalent method employing other specially designed polymers.32–35
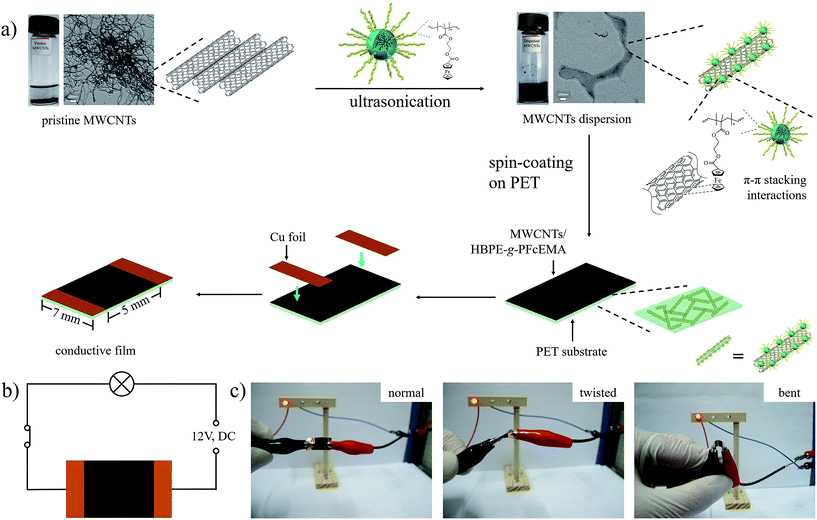
The dispersibility of the MWCNTs employing HBPE-g-PFcEMA was evaluated by the TEM technique. As shown in Fig. 3a, the pristine MWCNTs seemed to be in a bundle, due to the van der Waals interactions between the nanotubes. However, when the MWCNTs were dispersed by HBPE-g-PFcEMA, the majority of the individual MWCNTs were observed (Fig. 3b–f). Furthermore, the MWCNTs were found to be wrapped by HBPE-g-PFcEMA. These results indicated that HBPE-g-PFcEMA was an efficient dispersant for the dispersion of MWCNTs. The morphology of the MWCNTs/HBPE-g-PFcEMA composite on silica wafer was further studied by the SEM technique. Obvious clusters were observed in the pristine MWCNTs (Fig. S2a†), while individual MWCNTs wrapped by HBPE-g-PFcEMA were observed in the MWCNTs/HBPE-g-PFcEMA dispersion (Fig. S2b and c†). The MWCNTs were found to be buried in the HBPE-g-PFcEMA matrix when the mass ratio of HBPE-g-PFcEMA/MWCNTs was above 2.0.
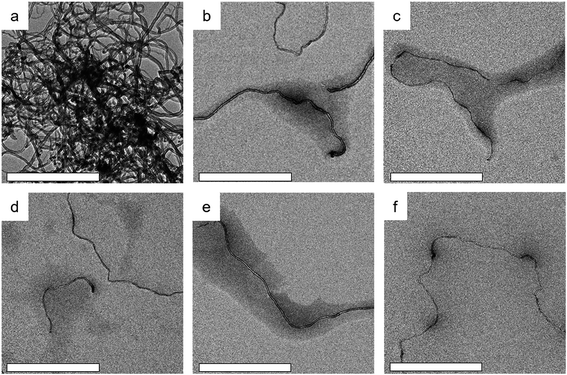 | ||
| Fig. 3 TEM images of pristine MWCNTs (a) and MWCNTs/HBPE-g-PFcEMA dispersion with different mass ratios of HBPE-g-PFcEMA/MWCNTs ((b) 0.5, (c) 1.0, (d) 2.0, (e) 5.0 and (f) 10.0). Scale bar: 500 nm. | ||
The interactions between HBPE-g-PFcEMA and the MWCNTs were studied by UV-vis spectroscopy. HBPE-g-PFcEMA showed a characteristic energy absorption band at 242 nm, which corresponded to the π–π* transition of the cyclopentadiene of ferrocene. On comparison with HBPE-g-PFcEMA, the absorbance of the MWCNTs/HBPE-g-PFcEMA dispersion at 242 nm was remarkably suppressed (Fig. 2c), which indicated the presence of π–π stacking interactions between the ferrocene of HBPE-g-PFcEMA and the aromatic rings of MWCNTs.25,26 We then proposed a conceivable mechanism for the dispersal of the MWCNTs in CHCl3 employing HBPE-g-PFcEMA (Fig. 4a), based on the experimental results discussed above. The pristine MWCNTs tend to be in a bundle due to the van der Waals interactions of the nanotubes. The bundled MWCNTs are de-bundled under ultrasonication. Meanwhile, the HBPE-g-PFcEMA is absorbed onto the surface of the MWCNTs, mainly through π–π stacking interactions between the ferrocene of HBPE-g-PFcEMA and the aromatic rings of the MWCNTs. The absorbed HBPE-g-PFcEMA then acts as a separator to prevent the aggregation of the de-bundled MWCNTs as well as providing the solubility of the MWCNTs. Thus, a stable MWCNTs/HBPE-g-PFcEMA dispersion is obtained.
Furthermore, the fabrication of a flexible MWCNTs/HBPE-g-PFcEMA composite conductive film by spin-coating the stable MWCNTs/HBPE-g-PFcEMA dispersion onto the polyethylene terephthalate (PET) substrate was explored (Fig. 4a). The thickness of the conductive film (6.85 μm) was obtained from an eddy current thickness meter. Then, the conductivity of the prepared film was measured by using the four-probe conductivity meter (Scheme S1†). As shown in Fig. S3,† the measured voltages were linearly correlated with the currents applied. The ratio of the voltage to current (3.42 kΩ) was obtained after linear fitting. The highest conductivity of the prepared conductive film was calculated to be 943.4 S cm−1, when the mass ratio of HBPE-g-PFcEMA/MWCNTs was 10.0. These results indicated that the prepared conductive film had good conductivity. Moreover, the prepared conductive film retained its good conductivity even after twisting or bending (Fig. 4c). Thus, the flexible film might find potential application in flexible electronics, such as flexible batteries36 and supercapacitors.37
Conclusion
In summary, a novel ferrocenyl hyper-branched polyethylene (HBPE-g-PFcEMA) was synthesized by a successive Pd-diimine-catalyzed CWP and ATRP process to disperse MWCNTs in CHCl3. It was found that the HBPE-g-PFcEMA had the ability to disperse MWCNTs efficiently, and the π–π stacking interactions between the ferrocene of HBPE-g-PFcEMA and the aromatic rings of the MWCNTs were confirmed. Furthermore, fabrication of a flexible conductive film via spin-coating the MWCNTs/HBPE-g-PFcEMA dispersion onto a PET substrate was explored. The conductive film showed good conductivity even after being twisted or bent. Thus, the ferrocenyl hyper-branched polyethylene reported here was shown to be an efficient dispersant for dispersing MWCNTs in CHCl3 and for preparing a flexible CNT-based conductive film, which might have potential application in flexible electronics.Acknowledgements
Financial supports from the National Natural Science Foundation of China (21272210, 21472168, 21372200 and 21411130187), the Science and Technology Program of Zhejiang Province (2013C24001 and 2013C31146), the Science and Technology Program of Ningbo (2013B6001), and the Science and Technology Innovation Team of Ningbo (2011B82002) are gratefully acknowledged.References
- L. Li, Z. Yu, W. Hu, C. Chang, Q. Chen and Q. Pei, Adv. Mater., 2011, 23, 5563–5567 CrossRef CAS PubMed.
- Z. Yu, L. Li, Q. Zhang, W. Hu and Q. Pei, Adv. Mater., 2011, 23, 4453–4457 CrossRef CAS PubMed.
- J. Lee, P. Lee, H. B. Lee, S. Hong, I. Lee, J. Yeo, S. S. Lee, T. S. Kim, D. Lee and S. H. Ko, Adv. Funct. Mater., 2013, 23, 4171–4176 CrossRef CAS.
- D. S. Hecht, L. Hu and G. Irvin, Adv. Mater., 2011, 23, 1482–1513 CrossRef CAS PubMed.
- D. Janas and K. K. Koziol, Nanoscale, 2014, 6, 3037–3045 RSC.
- J. Du, S. Pei, L. Ma and H. M. Cheng, Adv. Mater., 2014, 26, 1958–1991 CrossRef CAS PubMed.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS PubMed.
- N. G. Sahoo, S. Rana, J. W. Cho, L. Li and S. H. Chan, Prog. Polym. Sci., 2010, 35, 837–867 CrossRef CAS.
- J. N. Coleman, U. Khan, W. J. Blau and Y. K. Gun'ko, Carbon, 2006, 44, 1624–1652 CrossRef CAS.
- J. N. Coleman, U. Khan and Y. K. Gun'ko, Adv. Mater., 2006, 18, 689–706 CrossRef CAS.
- P. Liu, Eur. Polym. J., 2005, 41, 2693–2703 CrossRef CAS.
- D. Baskaran, J. W. Mays and M. S. Bratcher, Chem. Mater., 2005, 17, 3389–3397 CrossRef CAS.
- A. Zhang, M. Tang, J. Luan and J. Li, Mater. Lett., 2012, 67, 283–285 CrossRef CAS.
- D. M. Guldi, H. Taieb, G. M. A. Rahman, N. Tagmatarchis and M. Prato, Adv. Mater., 2005, 17, 871–875 CrossRef CAS.
- Y. J. Kang and T. A. Taton, J. Am. Chem. Soc., 2003, 125, 5650–5651 CrossRef CAS PubMed.
- Z. Iatridi and C. Tsitsilianis, Soft Matter, 2013, 9, 185–193 RSC.
- H. W. Lee, Y. Yoon, S. Park, J. H. Oh, S. Hong, L. S. Liyanage, H. Wang, S. Morishita, N. Patil, Y. J. Park, J. J. Park, A. Spakowitz, G. Galli, F. Gygi, P. H. S. Wong, J. B. H. Tok, J. M. Kim and Z. Bao, Nat. Commun., 2011, 2, 541 CrossRef PubMed.
- J. T. Sun, C. Y. Hong and C. Y. Pan, Polym. Chem., 2011, 2, 998–1007 RSC.
- L. Xu, Z. Ye, Q. Cui and Z. Gu, Macromol. Chem. Phys., 2009, 210, 2194–2202 CrossRef CAS.
- L. Xu, Z. Ye, S. Siemann and Z. Gu, Polymer, 2014, 55, 3120–3129 CrossRef CAS.
- K. Zhang, J. Wang, R. Subramanian, Z. Ye, H. Lu and Q. Yu, Macromol. Rapid Commun., 2007, 28, 2185–2191 CrossRef CAS.
- O. Osazuwa, K. Petrie, M. Kontopoulou, P. Xiang, Z. Ye and A. Docoslis, Compos. Sci. Technol., 2012, 73, 27–33 CrossRef CAS.
- K. Petrie, A. Docoslis, S. Vasic, M. Kontopoulou, S. Morgan and Z. Ye, Carbon, 2011, 49, 3378–3382 CrossRef CAS.
- Y. Zhao, L. Wang, A. Xiao and H. Yu, Prog. Polym. Sci., 2010, 35, 1195–1216 CrossRef CAS.
- X. Mao, G. C. Rutledge and T. A. Hatton, Langmuir, 2013, 29, 9626–9634 CrossRef CAS PubMed.
- Z. Deng, H. Yu, L. Wang and X. Zhai, J. Organomet. Chem., 2015, 791, 274–278 CrossRef CAS.
- X. Mao, F. Simeon, D. S. Achilleos, G. C. Rutledge and T. A. Hatton, J. Mater. Chem. A, 2013, 1, 13120–13127 CAS.
- A. Glidle, A. R. Hillman, K. S. Rydeo, E. L. Smith, J. Cooper, N. Gadegaard, J. R. P. Webster, R. Dalgliesh and R. Cubitt, Langmuir, 2009, 25, 4093–4103 CrossRef CAS PubMed.
- M. T. Sulak, O. Gokdogan, A. Gulce and H. Gulce, Biosens. Bioelectron., 2006, 21, 1719–1726 CrossRef PubMed.
- S. Bruckenstein, P. Krtil and A. R. Hillman, J. Phys. Chem. B, 1998, 102, 4994–5003 CrossRef CAS.
- Z. Deng, H. Yu, L. Wang, X. Zhai, Y. Chen and R. Sun, J. Organomet. Chem., 2015, 799–800, 273–280 CrossRef CAS.
- J. Zou, L. Liu, H. Chen, S. I. Khondaker, R. D. McCullough, Q. Huo and L. Zhai, Adv. Mater., 2008, 20, 2055–2060 CrossRef CAS.
- Y. Sabba and E. L. Thomas, Macromolecules, 2004, 37, 4815–4820 CrossRef CAS.
- C. S. Lovell, K. E. Wise, J.-W. Kim, P. T. Lillehei, J. S. Harrison and C. Park, Polymer, 2009, 50, 1925–1932 CrossRef CAS.
- Z. Zhang, Y. Che, R. A. Smaldone, M. Xu, B. R. Bunes, J. S. Moore and L. Zang, J. Am. Chem. Soc., 2010, 132, 14113–14117 CrossRef CAS PubMed.
- H. Lee, J. Yoo, J. Park, J. H. Kim, K. Kang and Y. S. Jung, Adv. Energy Mater., 2012, 2, 976–982 CrossRef CAS.
- J. Yun, D. Kim, G. Lee and J. S. Ha, Carbon, 2014, 79, 156–164 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01182a |
| This journal is © The Royal Society of Chemistry 2016 |