Influence of various chloride ion concentrations on silver nanoparticle transformations and effectiveness in surface enhanced Raman scattering for different excitation wavelengths†
Abstract
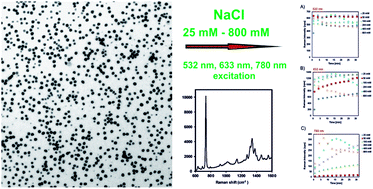
The effect of six various chloride ion concentrations (25, 50, 100, 200, 400, and 800 mM) on time-dependence and surface enhanced Raman scattering (SERS) signal intensity was investigated for silver nanoparticles (∼28 nm) with a high monodispersity and long time stability. The experiments were performed using three lasers with excitation wavelengths in the visible region (532 nm, 633 nm, 780 nm). Adenine was used as a model analyte. The treatment procedure, when the various sodium chloride solutions added to silver nanoparticles, led to an enhancement in the Raman signal at all studied concentration levels of sodium chloride. Nevertheless, low-concentration chloride ions differently influenced the time course of enhancement efficiency contrary to high-concentration chloride ions. The final concentration of chloride ions equal to 25 mM did not have any pronounced influence on the silver particle sizes and morphologies. The final concentration of chloride ions varying from 50 to 200 mM led to the etching and coalescence of silver nanoparticles. Higher concentrations of chlorides (400 mM) caused re-crystallization of primary silver nanoparticles to one order larger crystallites (400 nm). From the point of view of SERS, the time dependent profiles of Raman signal enhancement differ only slightly for all the used final concentrations of chloride ions when using excitation at 532 nm. On the contrary, for excitation wavelengths 633 nm and 780 nm, the time dependent profiles of Raman signal enhancement were very different when using above mentioned six various final concentrations of chloride ions.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...