Open Access Article
Open Access ArticleDiasteroselective multi-component assemblies from dynamic covalent imine condensation and metal-coordination chemistry: mechanism and narcissistic stereochemistry self-sorting†
Elena Badetti a,
Nadia Alessandra Carmo dos Santos
a,
Nadia Alessandra Carmo dos Santos a,
Francesca A. Scaramuzzo
a,
Francesca A. Scaramuzzo a,
Carlo Bravin
a,
Carlo Bravin a,
Klaus Wurst
a,
Klaus Wurst b,
Giulia Licini
b,
Giulia Licini a and
Cristiano Zonta
a and
Cristiano Zonta *a
*a
aDepartment of Chemical Sciences, University of Padova, Via Marzolo 1, 35131 Padova (PD), Italy. E-mail: cristiano.zonta@unipd.it
bInstitute of General, Inorganic and Theoretical Chemistry, University of Innsbruck, Innrain 80/82, 6020 Innsbruck, Austria
First published on 29th May 2018
Abstract
Self-assembly of a modified tris(2-pyridylmethyl)amine TPMA ligand, zinc(II) or cobalt(II) ions, and amino acids have been used effectively as stereo dynamic optical probes for the determination of the enantiomeric excess of free amino acids either using Electronic or Vibrational Circular Dichroism (CD and VCD). Herein, we report the mechanistic and stereochemical study of the self-assembly process which reveals a complex equilibrium in solution where even small variations in the experimental conditions can profoundly affect the final products of the reaction. In particular, variation on the metal stoichiometry switch give rises to an entirely enantio narcissistic self-assembly of the structure.
Introduction
One of the leading strategies for the development of complex functional nano-architectures is the use of dynamic covalent chemistry (DCC) in which imine condensation and metal-coordination work simultaneously.1 These two “orthogonal” thermodynamically driven association processes have successfully and effectively yield a large variety of compounds, ranging from simple complexes to topological structures.2 This approach has been effectively used for the development of methods that allow the determination of optical purity.3 In particular, the focus was driven towards the development of stereodynamic optical probes4 for the rapid determination of the enantiomeric excess (e.e.) of chiral compounds. In general, these systems are characterized by the presence of at least one labile stereogenic element which, after the addition of a chiral analyte, moves toward the formation of one preferential diastereoisomer. Most of the times, this information is translated into a signal by the use of optical techniques such as circular dichroism (CD).5Recently, we have been working with tris(2-pyridylmethyl)amine (TPMA) metal complexes both in catalysis6 and supramolecular chemistry.7 Within these studies, we reported a series of novel molecular architectures that can be used for the determination of the e.e. of free amino acids (Fig. 1).7a,7b,8 More in detail, the mix of a modified TPMA zinc complex with one equivalent of the desired amino acid resulted in the almost exclusive formation of the dinuclear architecture 1-aaa. The mixture was used without further purification for CD measurements. The obtained curves directly correlate with the e.e. and specular curves were obtained switching the configuration of the amino acids. Beside the capability of the system to be effective with all natural amino acids, it displayed absorptions which were characteristic of the amino acidic side chain, differentiating clearly the aliphatic or aromatic nature of the backbone. TD-DFT studies have shown that CD signals arise by the helical arrangement of the ligand around the metal, and by the exciton coupling of the atropoisomeric biarylic systems. In addition, from studies on the 1-aaa complex stability we could identify the structure 2-aaa, in which an extra-metal get coordinated by the cleft formed between the two amino acids.7b
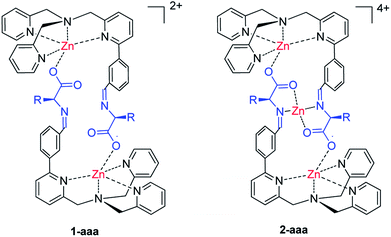 | ||
| Fig. 1 Dinuclear 1-aaa and trinuclear 2-aaa self-assembled structures used as probes for the determination of e.e. of amino acids. The counteranions are perchlorate. | ||
These initial findings have driven our interest towards the investigation of the whole process taking place in solution, with the two-fold intention of better understanding the self-assembly mechanism and elucidating how the stereochemistry dictates products distribution. The elucidation of the equilibrium present in solution, when imine DCC is used in the presence of metals, highlights that increasing system complexity leads to sharp changes in products distribution and stereochemistry from small variations of the chemical parameters.
Results and discussion
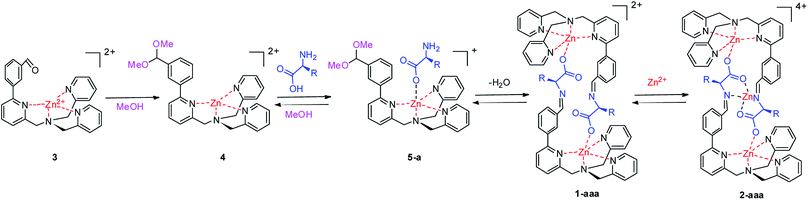
In order to shed light on the mechanism behind the formation of bi- and tri-nuclear dimers, a step-by-step analysis has been performed evaluating how different thermodynamic, kinetic and stereochemical parameters are affecting the assembly formation and, consequently, the dichroic signal. Starting from complex 3, the delicate interplay of all the components present in the reaction mixture was revealed.Intermediates in the self-assembly process
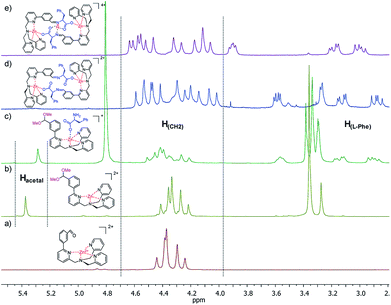
The complete conversion of 3 to 4 requires two days at room temperature but only 4 hours at 60 °C. The reaction can be easily monitored by 1H-NMR, following the disappearance of the aldehydic signal of 3 at 10.02 ppm and the appearance of the signal of acetal 4 at 5.37 ppm (Fig. 2b). Acetal formation is also confirmed by 2D-NMR HMQC experiment which correlates acetal proton signal with the characteristic 13C signal at 105 ppm.
Upon adding the amino acid L-Phe to acetal 4, the formation of a new species is instantaneously observed (Fig. 2c). It is possible to ascribe this structure to the adduct 5-L-Phe in which the amino acidic carboxylate binds to the zinc centre. This compound is highly stable in solution as variation of 1H NMR signals are not observed: (i) increasing the temperature, (ii) leaving the compound in solution for weeks, (iii) adding extra equivalents of amino acid or (iv) extra zinc salt to the sample. In these cases, the intensity of the acetal signal remains constant. Moreover, 1H NMR highlights a broadness of the picolyl CH2 signals (4.2–4.5 ppm) slightly different from the parent compounds 3 and 4 (Fig. 2). This observation can indicate the preferential formation of one of the two possible diastereoisomers generated by the helical conformations adopted by the ligand around the metal. However, the absence of any meaningful dichroic signal suggests a weak preference over one diastereoisomer.
In this compound, the presence of six doublets (J ∼ 16 Hz) between δ = 3.9 and 4.6 ppm, combined with VT-NMR experiments,7b suggest that the picolyl methylene hydrogens are blocked in a single helical conformation as expressed by the wide separation of the signals. Further confirmation also comes from ESI-MS experiments that show the characteristic isotopic pattern corresponding to the dinuclear zinc complex.
Stereochemical analysis of 1-aaa and 2-aaa assembly formation and self-sorting experiment
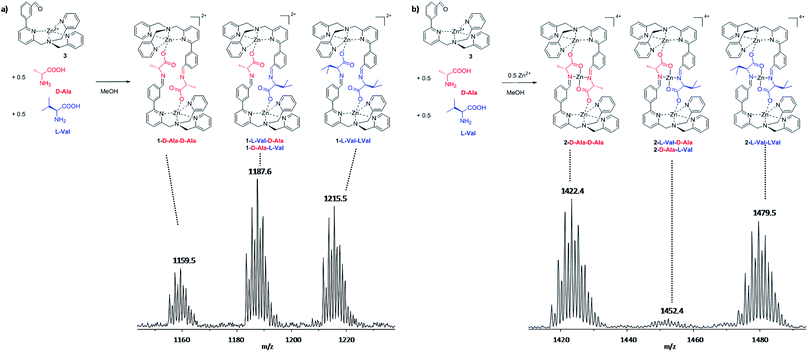
As shown in our previous work,7a,7b when 1-Phe is formed varying amino acid e.e., a linear response of the CD signal is observed. This linearity, besides constituting the bases for a probe, does not give information about the stereochemical recognition process along the formation of a chiral dimeric structure. In other words, two types of structures can be formed starting from a racemic mixture of the amino acid: (i) homochiral, where both the amino acids incorporated in the structure have the same configuration (C2 symmetry homo-narcissistic assembly, CD active) or (ii) heterochiral, where the two amino acids have opposite configuration (S2 symmetry hetero-narcissistic assembly, CD inactive).9 Due to their symmetry these two systems can not be identified using NMR and on the other hand, the linearity in CD response by itself does not give information on which type of structure is preferentially formed. In other word, independently by the assembly mechanism a linear correlation between e.e. and CD response is obtained (for a detailed explanation on how e.e. and CD correlates see ESI† paragraph 3).In order to investigate which type of assembly was more stable in solution, we devised an experiment where the dinuclear 1 and trinuclear 2 complexes were prepared from a solution containing a solution of L-Val and D-Ala, two pseudo-racemic amino acids that have similar side chains.
Using the pseudo-racemic mixture, the dinuclear 1-aaa have been obtained following our standard procedure. The resulting mixture was analysed using ESI-MS experiments to understand the stereochemistry of amino acids incorporation in the self-sorting experiment (Fig. 3a and ESI† Section 4). Assuming a similar response factor for the pseudo-diastereoisomers in the ESI-MS experiment,7g,10 the dinuclear 1-aaa assembly displays a small tendency to prefer the formation of the heterochiral structure. However, the three pseudo-diastereoisomers 1-D-Ala-D-Ala![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-L-Val-D-Ala + 1-D-Val-L-Ala:
1-L-Val-D-Ala + 1-D-Val-L-Ala:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-L-Val-L-Val are close to the statistical 1
1-L-Val-L-Val are close to the statistical 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (measured 1
1 (measured 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4:
4:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).
The more stable pseudo-diastereoisomer in solution, in the case of the dinuclear system 1-aaa, has the amino acids with opposite configuration. The same experiment was repeated adding 0.5 equivalents of zinc(II) perchlorate. In this case, we are in the situation were the trinuclear 2-aaa systems is formed. As it can be seen from ESI-MS (Fig. 3b), the 2-aaa shows a complete preference toward the formation of the homochiral assembly 2-D-Ala-D-Ala and the 2-L-Val-L-Val. In other words, the presence of the extra metal centre drives the system toward the formation of the enantio-narcissistic structure. Most probably, the metal adds an extra constriction to the amino acidic cleft which allows only the selection of identical amino acids.
Interestingly, another proof of the enantio narcissistic behaviour of the trinuclear assembly came from the X-ray analysis of crystals grown up when a racemic mixture of L/D-Phe was used in the synthesis (Fig. 4). The diffraction revealed a racemate which had in the same unit cell only the two enantiomeric homochiral narcissistic assemblies.
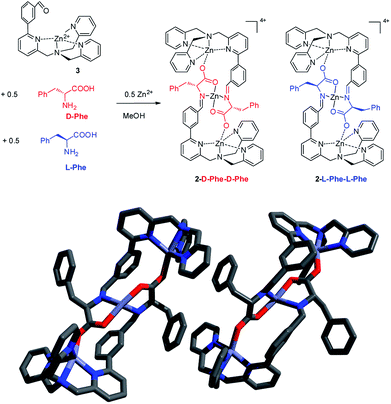 | ||
| Fig. 4 X-ray crystal structure of 2-Phe starting from the racemic Phe. Both 2-D-Phe-D-Phe and the 2-L-Phe-L-Phe are present in the same unit cell. The counteranions are perchlorates. | ||
Conclusions
In this study, we have shown that in our molecular architectures, the combination of coordination chemistry and DCC results in a delicate and intricate system which can be finely biased by external stimuli of the thermodynamic equilibria. In detail, solvent, molecular sieves, excess of components, temperature, and stereochemistry of the components, can have a profound impact in the outcome of a dynamic library. Even at a first sight this seems a very complicated system, the deep knowledge acquired in each step of the process allows to drive the synthesis towards the desired products. In other words, we were able not only to set-up an analytical method for the easy and fast detection of amino acid e.e., but also, through the synthesis and characterization of the pure compounds involved in the dynamic process, to gather information about the metal induced self-sorting of the self-assembly which has revealed the formation of an enantio narcissistic assembly.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Italian MIUR (PRIN-2010-11 2010CX2TLM_002), from the University of Padova (PRAT-CPDA153122, Cariparo Dottorati Internazionali NACS). Useful discussions have been carried within the frame of Cost Action CM1304 – Emergence and Evolution of Complex Chemical Systems.Notes and references
- (a) J. Bunzen, J. Iwasa, P. Bonakdarzadeh, E. Numata, K. Rissanen, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2012, 51, 3161–3163 CrossRef PubMed; (b) Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef PubMed; (c) D. A. Leigh, R. G. Pritchard and A. J. Stephens, Nat. Chem., 2014, 6, 978–982 CrossRef PubMed; (d) V. Marcos, A. J. Stephens, J. Jaramillo-Garcia, A. L. Nussbaumer, S. L. Woltering, A. Valero, J.-F. Lemonnier, I. J. Vitorica-Yrezabal and D. A. Leigh, Science, 2016, 352, 1555–1559 CrossRef PubMed; (e) K. S. Chichak, S. J. Cantrill, A. R. Pease, S.-H. Chiu, G. W. V. Cave, J. L. Atwood and J. F. Stoddart, Science, 2004, 304, 1308–1312 CrossRef PubMed; (f) J.-N. Rebilly, B. Colasson, O. Bistri, D. Over and O. Reinaud, Chem. Soc. Rev., 2015, 44, 467–489 RSC; (g) S. H. A. M. Leenders, R. G. Doria, B. de Bruin and J. N. H. Reek, Chem. Soc. Rev., 2015, 44, 433–448 RSC; (h) S. Durot, J. Taesch and V. Heitz, Chem. Rev., 2014, 114, 8542–8578 CrossRef PubMed; (i) T.-Z. Xie, K. J. Endres, Z. Guo, J. M. Ludlow, C. N. Moorefield, M. J. Saunders, C. Wesdemiotis and G. R. Newkome, J. Am. Chem. Soc., 2016, 138, 12344–12347 CrossRef PubMed; (j) J. Atcher, J. Bujons and I. Alfonso, Chem. Commun., 2017, 53, 4274–4277 RSC; (k) J. Atcher, A. Moure, J. Bujons and I. Alfonso, Chem.–Eur. J., 2015, 21, 6869–6878 CrossRef PubMed; (l) A. M. Valdivielso, F. Puig-Castellví, J. Atcher, J. Solà, R. Tauler and I. Alfonso, Chem.–Eur. J., 2017, 23, 10702 CrossRef; (m) M. Ciaccia, I. Tosi, L. Baldini, R. Cacciapaglia, L. Mandolini, S. Di Stefano and C. A. Hunter, Chem. Sci., 2015, 6, 144–151 RSC; (n) L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef PubMed; (o) D. Zamora-Olivares, T. S. Kaoud, K. N. Dalby and E. V. Anslyn, J. Am. Chem. Soc., 2013, 135, 14814–14820 CrossRef PubMed; (p) Z. Kostereli, R. Scopelliti and K. Severin, Chem. Sci., 2014, 5, 2456–2460 RSC; (q) T. Takeuchi and S. Matile, Chem. Commun., 2013, 49, 19–29 RSC; (r) E. Faggi, C. Vicent, S. V. Luis and I. Alfonso, Org. Biomol. Chem., 2015, 13, 11721–11731 RSC; (s) E. Faggi, A. Moure, M. Bolte, C. Vicent, S. V. Luis and I. Alfonso, J. Org. Chem., 2014, 79, 4590–4601 CrossRef PubMed.
- (a) M. Mastalerz, Angew. Chem., Int. Ed., 2010, 49, 5042–5053 CrossRef PubMed; (b) A. Granzhan, C. Schouwey, T. Riis-Johannessen, R. Scopelliti and K. Severin, J. Am. Chem. Soc., 2011, 133, 7106–7115 CrossRef PubMed; (c) T. Mitra, K. E. Jelfs, M. Schmidtmann, A. Ahmed, S. Y. Chong, D. J. Adams and A. I. Cooper, Nat. Chem., 2013, 5, 276–281 CrossRef PubMed; (d) T. K. Ronson, C. Giri, N. Kodiah Beyeh, A. Minkkinen, F. Topić, J. J. Holstein, K. Rissanen and J. R. Nitschke, Chem.–Eur. J., 2013, 19, 3374–3382 CrossRef PubMed; (e) S. Zarra, D. M. Wood, D. A. Roberts and J. R. Nitschke, Chem. Soc. Rev., 2015, 44, 419–432 RSC; (f) M. J. Barrell, A. G. Campaña, M. von Delius, E. M. Geertsema and D. A. Leigh, Angew. Chem., Int. Ed., 2011, 50, 285–290 CrossRef PubMed.
- (a) D. Leung, S. O. Kang and E. V. Anslyn, Chem. Soc. Rev., 2012, 41, 448–479 RSC; (b) G. Pescitelli, L. Di Bari and N. Berova, Chem. Soc. Rev., 2014, 43, 5211–5233 RSC; (c) N. Berova, L. Di Bari and G. Pescitelli, Chem. Soc. Rev., 2007, 36, 914–931 RSC; (d) C. Wolf and K. W. Bentley, Chem. Soc. Rev., 2013, 42, 5408–5424 RSC.
- (a) S. Hayashi, M. Yotsukura, M. Noji and T. Takanami, Chem. Commun., 2015, 51, 11068–11071 RSC; (b) P. Metola, E. V. Anslyn, T. D. James and S. D. Bull, Chem. Sci., 2012, 3, 156–161 RSC; (c) Z. A. De los Santos and C. Wolf, J. Am. Chem. Soc., 2016, 138, 13517–13520 CrossRef PubMed; (d) G. Bian, S. Yang, H. Huang, H. Zong, L. Song, H. Fan and X. Sun, Chem. Sci., 2016, 7, 932–938 RSC; (e) A. Akdeniz, T. Minami, S. Watanabe, M. Yokoyama, T. Ema and P. Anzenbacher Jr, Chem. Sci., 2016, 7, 2016–2022 RSC; (f) Z. A. De los Santos, R. Ding and C. Wolf, Org. Biomol. Chem., 2016, 14, 1934–1939 RSC; (g) C. Ni, D. Zha, H. Ye, Y. Hai, Y. Zhou, E. Anslyn and L. You, Angew. Chem., Int. Ed., 2018, 57, 1300–1305 CrossRef PubMed.
- (a) L. You, J. S. Berman and E. V. Anslyn, Nat. Chem., 2011, 3, 943–948 CrossRef PubMed; (b) C.-Y. Lin, S. Lim and E. V. Anslyn, J. Am. Chem. Soc., 2016, 138, 8045–8047 CrossRef PubMed; (c) L. You, J. S. Berman, A. Lucksanawichien and E. V. Anslyn, J. Am. Chem. Soc., 2012, 134, 7126–7134 CrossRef PubMed; (d) L. You, G. Pescitelli, E. V. Anslyn and L. Di Bari, J. Am. Chem. Soc., 2012, 134, 7117–7125 CrossRef PubMed; (e) H. H. Jo, X. Gao, L. You, E. V. Anslyn and M. J. Krische, Chem. Sci., 2015, 6, 6747–6753 RSC; (f) L. A. Joyce, M. S. Maynor, J. M. Dragna, G. M. da Cruz, V. M. Lynch, J. W. Canary and E. V. Anslyn, J. Am. Chem. Soc., 2011, 133, 13746–13752 CrossRef PubMed; (g) J. Zhang, W. Sheng, H. Gholami, T. Nehira and B. Borhan, Chirality, 2017, 1–6 Search PubMed; (h) H. Gholami, M. Anyika, J. Zhang, C. Vasileiou and B. Borhan, Chem.–Eur. J., 2016, 22, 9235–9239 CrossRef PubMed; (i) M. Tanasova, M. Anyika and B. Borhan, Angew. Chem., Int. Ed., 2015, 54, 4274–4278 CrossRef PubMed; (j) M. Anyika, H. Gholami, K. D. Ashtekar, R. Acho and B. Borhan, J. Am. Chem. Soc., 2014, 136, 550–553 CrossRef PubMed; (k) H. Gholami, J. Zhang, M. Anyika and B. Borhan, Org. Lett., 2017, 19, 1722–1725 CrossRef PubMed; (l) D. Zhang, J. R. Cochrane, S. Di Pietro, L. Guy, H. Gornitzka, J.-P. Dutasta and A. Martinez, Chem.–Eur. J., 2017, 23, 6495–6498 CrossRef PubMed; (m) D. Zhang, B. Bousquet, J.-C. Mulatier, D. Pitrat, M. Jean, N. Vanthuyne, L. Guy, J.-P. Dutasta and A. Martinez, J. Org. Chem., 2017, 82, 6082–6088 CrossRef PubMed; (n) P. Zardi, K. Wurst, G. Licini and C. Zonta, J. Am. Chem. Soc., 2017, 139, 15616–15619 CrossRef PubMed.
- (a) M. Natali, E. Badetti, E. Deponti, M. Gamberoni, F. A. Scaramuzzo, A. Sartorel and C. Zonta, Dalton Trans., 2016, 45, 14764–14773 RSC; (b) N. A. Carmo dos Santos, E. Badetti, M. Natali, K. Wurst, G. Licini and C. Zonta, Dalton Trans., 2017, 46, 16455–16464 RSC; (c) N. A. Carmo dos Santos, F. Lorandi, E. Badetti, K. Wurst, A. A. Isse, A. Gennaro, G. Licini and C. Zonta, Polymer, 2017, 128, 169–176 CrossRef.
- (a) F. A. Scaramuzzo, G. Licini and C. Zonta, Chem.–Eur. J., 2013, 19, 16809–16813 CrossRef PubMed; (b) E. Badetti, K. Wurst, G. Licini and C. Zonta, Chem.–Eur. J., 2016, 22, 6515–6518 CrossRef PubMed; (c) R. Berardozzi, E. Badetti, N. A. Carmo dos Santos, K. Wurst, G. Licini, G. Pescitelli, C. Zonta and L. Di Bari, Chem. Commun., 2016, 52, 8428–8431 RSC; (d) F. A. Scaramuzzo, E. Badetti, G. Licini and C. Zonta, Eur. J. Org. Chem., 2017, 11, 1438–1442 CrossRef; (e) C. Bravin, E. Badetti, F. A. Scaramuzzo, G. Licini and C. Zonta, J. Am. Chem. Soc., 2017, 139, 6456–6460 CrossRef PubMed; (f) N. A. Carmo dos Santos, E. Badetti, G. Licini, S. Abbate, G. Longhi and C. Zonta, Chirality, 2018, 30, 65–73 CrossRef PubMed; (g) C. Bravin, E. Badetti, R. Puttreddy, F. Pan, K. Rissanen, G. Licini and C. Zonta, Chem.–Eur. J., 2018, 24, 2936–2943 CrossRef PubMed.
- For other systems for e.e. detection of amino-acids see: (a) F. Biedermann and W. M. Nau, Angew. Chem., Int. Ed., 2014, 53, 5694–5699 CrossRef PubMed; (b) E. G. Shcherbakova, V. Brega, T. Minami, S. Sheykhi, T. D. James and P. Anzenbacher Jr, Chem.–Eur. J., 2016, 22, 10074–10080 CrossRef PubMed; (c) S. Shinoda, K. Terada and H. Tsukube, Chem.–Asian J., 2012, 7, 400–405 CrossRef PubMed; (d) K. W. Bentley, Y. G. Nam, J. M. Murphy and C. Wolf, J. Am. Chem. Soc., 2013, 135, 18052–18055 CrossRef PubMed; (e) N. Kameta, M. Masuda and T. Shimizu, Chem. Commun., 2015, 51, 11104–11107 RSC; (f) K. W. Bentley, P. Zhang and C. Wolf, Sci. Adv., 2016, 2, e1501162 Search PubMed; (g) K. W. Bentley and C. Wolf, J. Org. Chem., 2014, 79, 6517–6531 CrossRef PubMed.
- (a) A. Wu and L. Isaacs, J. Am. Chem. Soc., 2003, 125, 4831–4835 CrossRef PubMed; (b) M. L. Saha and M. Schmittel, Org. Biomol. Chem., 2012, 10, 4651–4684 RSC.
- Due to a similar structure and charge of the measured peak we assume a similar response factor for the assemblies. (a) S. P. Black, D. M. Wood, F. B. Schwarz, T. K. Ronson, J. J. Holstein, A. R. Stefankiewicz, C. A. Schalley, J. K. M. Sanders and J. R. Nitschke, Chem. Sci., 2016, 7, 2614–2620 RSC; (b) E. Leize, A. Jaffrezic and A. Van Dorsselaer, J. Mass Spectrom., 1996, 31, 537–544 CrossRef; (c) P. N. W. Baxter, R. G. Khoury, J.-M. Lehn, G. Baum and D. Fenske, Chem. – Eur. J., 2000, 6, 4140–4148 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1583270. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra03989e |
| This journal is © The Royal Society of Chemistry 2018 |