Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and biological activities of novel trifluoromethylpyridine amide derivatives containing sulfur moieties†
S. X. Guo‡
,
F. He‡,
A. L. Dai,
R. F. Zhang,
S. H. Chen and
J. Wu *
*
State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Research and Development Center for Fine Chemicals, Guizhou University, Huaxi District, Guiyang 550025, P. R. China. E-mail: wujian2691@126.com; jwu6@gzu.edu.cn
First published on 28th September 2020
Abstract
A series of trifluoromethylpyridine amide derivatives containing sulfur moieties (thioether, sulfone and sulfoxide) was designed and synthesized. Their antibacterial activities against Xanthomonas oryzae pv. oryzae (Xoo), Ralstonia solanacearum (R. solanacearum) and insecticidal activities against P. xylostella were evaluated. Notably, the half-maximal effective concentration (EC50) value of sulfone-containing compound F10 is 83 mg L−1 against Xoo, which is better than that of commercial thiodiazole copper (97 mg L−1) and bismerthiazol (112 mg L−1). Thioether-containing compounds E1, E3, E5, E6, E10, E11 and E13 showed much higher activities against R. solanacearum with the EC50 value from 40 to 78 mg L−1, which are much lower than that of thiodiazole copper (87 mg L−1) and bismerthiazol (124 mg L−1). Generally, most of the sulfone-containing compounds and sulfoxide-containing compounds showed higher activities against Xoo than that of the corresponding thioether-containing compound, but most of the thioether-containing compounds contributed higher antibacterial activities against R. solanacearum. Furthermore, title compounds E3, E11, E24 and G2 showed good insecticidal activities of 75%, 70%, 70% and 75%, respectively.
1 Introduction
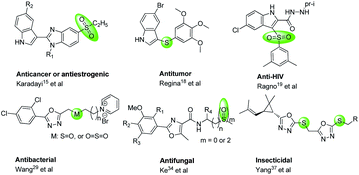
Crop diseases caused by bacteria, fungi, viruses, nematodes and oomycetes have posed a huge challenge for crop production, so that it is hard to provide sufficient food for the growing population.1,2 Particularly, rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae (Xoo) can reduce rice yields by 80%.3,4 Tobacco bacterial wilt caused by Ralstonia solanacearum (R. solanacearum), is another devastating disease.5 Pesticides play a crucial role in controlling crop diseases for agricultural cultivating systems, rapidly increasing the crop yields and food production.1,6 However, along with the resistance and cross resistance, the efficiencies of many pesticides have gradually reduced. Currently, because of the potential environmental, ecological and health risks, some pesticides have been gradually banned and withdrawn from the market. For example, bismerthiazol, used as a bactericide against rice bacterial blight, was banned by the Ministration of Agriculture, P. R. China due to its harmful effects towards some creatures. Consequently, the development of eco-friendly pesticides with novel action modes is urgently needed.Sulfur element is a crucial part of proteins and amino acids. It can be found in many secondary metabolites in living organisms, and exits also in many bio-active compounds (Fig. 1) with broaden biological activities,7–14 especially using for anti-cancer,15–18 anti-HIV,19,20 and treating acid-related disorder,21 and also using for the inhibitors of topoisomerase 1,22 anhydrase II,23 mutated B-Raf,24,25 cyclooxygenase-2.26,27 Particularly, compounds containing thioether, sulfone or sulfoxide moieties could exhibit significantly anti-bacterial,28–32 anti-fungal,33,34 herbicidal35 and insecticidal36,37 activities for crop protection. Thus, sulfur-containing molecules show promising properties for drug design and medicinal chemistry.38–40
Over decades, trifluoromethylpyridine ring, a special fluorinated nitrogen heterocycle, has been a hot spot for the creation of novel pesticides.41–43 For the period 2000–2017, among a total 166 ISO common name proposals, 10 (6%) contain a trifluoromethylpyridine, including 3 fungicides, 2 herbicides and 5 insecticides.43 Our previous works44–46 also revealed that some compounds containing trifluoromethyl pyridine showed excellent anti-virus, anti-bacterial and insecticidal activities.
Consequently, considering the concepts mentioned above, this work focused on the synthesis of trifluoromethylpyridine amide derivatives containing the thioether, sulfoxide or sulfone substructure, and their biological evaluation. Their primary structure–activity relationship for these novel trifluoromethylpyridine amide derivatives was also discussed.
2 Results and discussion
2.1 Design
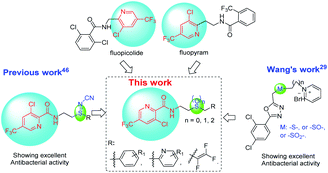
The commercial fungicides such as fluopicolide and fluopyram (Fig. 2) are typical trifluoromethylpyridine derivatives.43 Our previous work has revealed that trifluoromethyl pyridinamide derivatives containing a structure of “–S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CN” showed significantly bactericidal activities.46 Wang and co-workers29 has reported that some novel sulfur contained compounds can be used as potential antibacterial agents.29 Thus as shown in Fig. 2, this work sought to modify the previous structure through changing the “–S
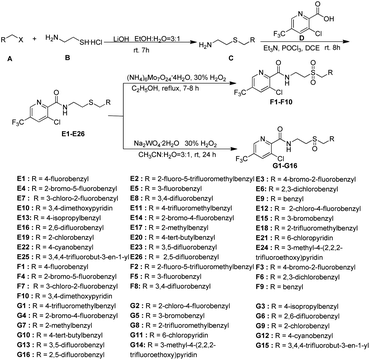
N–CN” showed significantly bactericidal activities.46 Wang and co-workers29 has reported that some novel sulfur contained compounds can be used as potential antibacterial agents.29 Thus as shown in Fig. 2, this work sought to modify the previous structure through changing the “–S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CN” to “–S–”, “–SO2–” or “–SO–” to obtain a series of novel thioether-containing compounds E1–E26. Then, the chemoselectivities of sulfone-containing compounds F1–F10 and sulfoxide-containing compounds G1–G16, were obtained by the oxidation of compounds E1–E26 in different conditions (Scheme 1). Their antibacterial activities against Xoo and R. solanacearum, and insecticidal activities against P. xylostella were evaluated. Desirably, some synthesized compounds could show higher antibacterial activities than that of commercialized thiodiazole copper and bismerthiazol. It's the first time that trifluoromethylpyridine amide derivatives containing the sulfur moiety were designed and synthesized for anti-bacterial and insecticidal applications.
N–CN” to “–S–”, “–SO2–” or “–SO–” to obtain a series of novel thioether-containing compounds E1–E26. Then, the chemoselectivities of sulfone-containing compounds F1–F10 and sulfoxide-containing compounds G1–G16, were obtained by the oxidation of compounds E1–E26 in different conditions (Scheme 1). Their antibacterial activities against Xoo and R. solanacearum, and insecticidal activities against P. xylostella were evaluated. Desirably, some synthesized compounds could show higher antibacterial activities than that of commercialized thiodiazole copper and bismerthiazol. It's the first time that trifluoromethylpyridine amide derivatives containing the sulfur moiety were designed and synthesized for anti-bacterial and insecticidal applications.
2.2 Chemistry
According to the reported methods,29,47,48 the title compounds E1–E26, F1–F10 and G1–G16 could be easily obtained as shown in Scheme 1. The thioether-containing compounds E1–E26 were firstly synthesized via two steps. Firstly, haloalkane (A) was treated with aminoethyl mercaptan (B) using LiOH as base and the mixture of ethanol and H2O in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as solvent under room temperature (rt) to obtain differently substituted 2-(ethylthio)amines (C).29,47,48 After being activated by POCl3, 3-chloro-5-(trifluoromethyl)picolinic acid (D) condensed with intermediate C to obtain thioether-containing compounds E1–E26.29,47,48 Thioether-containing compounds E1–E10 were then converted to corresponding sulfone-containing compounds F1–F10 in the presence of ammonium molybdate, 30% H2O2 as oxidant and ethanol as solvent at 100 °C.47,48 Different with the synthesis of sulfone compounds F1–F10, sulfoxide-containing compounds G1–G16 were obtained through the oxidation of E11–E26 in the presence of sodium tungstate dehydrate, 30% H2O2 as oxidant and the mixture of acetonitrile and water in a ratio of 3
1 as solvent under room temperature (rt) to obtain differently substituted 2-(ethylthio)amines (C).29,47,48 After being activated by POCl3, 3-chloro-5-(trifluoromethyl)picolinic acid (D) condensed with intermediate C to obtain thioether-containing compounds E1–E26.29,47,48 Thioether-containing compounds E1–E10 were then converted to corresponding sulfone-containing compounds F1–F10 in the presence of ammonium molybdate, 30% H2O2 as oxidant and ethanol as solvent at 100 °C.47,48 Different with the synthesis of sulfone compounds F1–F10, sulfoxide-containing compounds G1–G16 were obtained through the oxidation of E11–E26 in the presence of sodium tungstate dehydrate, 30% H2O2 as oxidant and the mixture of acetonitrile and water in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as solvent at rt.47,48
1 as solvent at rt.47,48
The title thioether-containing compounds E1–E26, sulfone-containing compounds F1–F10 and sulfoxide-containing compounds G1–G16 were characterized by the 1H NMR, 19F NMR, 13C NMR and HR-MS. Taking sulfone-containing compound F1 as an example, in the 1H NMR spectrum, the proton near “N” atom of trifluoromethylpyridine ring appeared as a doublet at δ 8.72 ppm. The proton of –CONH– was split into a triplet at δ 8.40 ppm. Another proton of trifluoromethylpyridine ring appeared also as a doublet at δ 8.07. The four protons of benzene ring were characterized as two multiples at δ 7.33–7.23 and δ 7.15–7.00. The protons of methylene near the benzene ring appeared as a quartet at δ 4.03. The protons of methylene near the carbonyl appeared as multiple at δ 3.11–2.77, and the protons of methylene appeared at δ 3.96 as double doublets. In 13C NMR spectrum, the carbons near “–CF3” or “F” were all split into quartets due to the coupling coefficients of “F”. For instance, the carbon of “–CF3” group was split into a quartet at δC 122.1 ppm with the coupling constant of (1JF–C) 273.3 Hz. Carbon at the position 5 in the pyridine, was split into another quartet with coupling constant of 34.1 Hz at δC 129.4. Two carbons at the position 4 and 6 of trifluoromethylpyridine, were also split into two quartets with smaller coupling constants. Other carbons near the atom “F” could also be split with different coupling constants. In the 19F NMR spectra, the fluorine of “–CF3” appeared at the shift of −62.57 ppm, and the fluorine on the benzene ring appeared at the shift of −112.80 ppm.
2.3 Preliminary in vitro antibacterial activity test
According to the reported method,49 the method of turbidity was adopted to evaluate the antibacterial activities of title compounds against Xanthomonas oryzae pv. oryzae (Xoo), Ralstonia solanacearum (R. solanacearum). The commercial thiodiazole copper (TC) and bismerthiazol (BT) were used as the positive controls. The results shown in Table 1 revealed that some of the synthesized compounds showed higher activities against Xoo and R. solanacearum than that of commercial bactericides at the concentration of 100 and 50 mg L−1. For example, at the concentration of 50 mg L−1, compounds E20 (R is 4-tert-butylbenzyl), E15 (R is 3-bromobenzyl), E9 (R is benzyl), E16 (R is 2,6-difluorobenzyl), E13 (R is 4-isopropylbenzyl) and E24 (R is 3-methyl-4-(2,2,2-trifluoroethoxy)pyridine) showed good activities against Xoo of 35%, 35%, 33%, 33%, 32% and 31% respectively, which are close to that of BT (31%). Desirably, when two electron withdraw groups, 3,4-dimethoxypyridine, substituted in the phenyl, sulfone-containing compound F10 exhibited the highest activities of 53% (100 mg L−1) and 42% (50 mg L−1) against Xoo, which are slightly higher than that of TC (53%, 39%) and BT (51%, 31%). Sulfone-containing compounds F1–F10 showed the different activities against Xoo as follows: 3,4-dimethoxypyridin (F10) > benzyl (F9) > 4-bromo-2-fluorobenzyl (F3) > 4-fluorobenzyl (F1) > 3-fluorobenzyl (F5) > 2,3-dichlorobenzyl (F6) > 2-fluoro-5-trifluoromethylbenzyl (F2) > 2-bromo-5-fluorobenzyl (F4) > 3-chloro-2-fluorobenzyl (F7) > 3,4-difluorobenzyl (F8). Among sulfone-containing compounds, compounds F3 (31%), F5 (31%) and F9 (32%) showed activities of 31%, 31% and 32% respectively against Xoo at 50 mg L−1, which are close to than that of BT (31%). Among sulfoxide-containing compounds, compounds G5 where R is 3-bromobenzyl and G16 where R is 2,5-difluorobenzyl showed activities of 32% and 35%, respectively against Xoo at 50 mg L−1, which are closed to that of BT (31%). The majority of oxidized compounds (sulfone or sulfoxide-containing compounds) could show higher activities against Xoo, compared with the thioether-containing compounds. For example, oxidizing thioether-containing compounds E3 (9%), E4 (13%), E7 (7%) and E17 (4%) to corresponding F3 (47%), F4 (32%), F7 (31%) and G7 (36%) could provide 38%, 26%, 24% and 32% higher activities against Xoo at 100 mg L−1, respectively. However, compounds E8 (32%), E22 (42%) and E24 (38%) showed higher activities against Xoo than that of corresponding oxidized compounds F8 (19%), G9 (18%) and G24 (28%). Thioether-containing compounds E13 (39%), E19 (44%), E20 (43%) and E23 (42%) showed slightly higher activities against Xoo at 100 mg L−1 than that of corresponding oxidized compounds G3 (35%), G9 (37%), G10 (33%) and G13 (39%). The rest of oxidized compounds owned higher activities against Xoo than that of corresponding thioether-containing compounds. Regarding R. solanacearum, thioether-containing compounds E1 (57%, 54%), E3 (53%, 44%), E5 (64%, 50%), E6 (67%, 52%), E10 (62%, 45%), E11 (54%) and E13 (63%, 56%) exhibited higher activities than that of TC (51%, 40%) and much higher activities than that of BT (38%, 17%) at 100 or 50 mg L−1. According to their activities, the R groups of them can be sorted as follows: 2,3-dichlorobenzyl (E6) > 3-fluorobenzyl (E5) > 4-isopropylbenzyl (E13) > 3,4-dimethoxypyridin (E10) > 4-fluorobenzyl (E1) > 4-trifluoromethylbenzyl (E11) > 4-bromo-2-fluorobenzyl (E3). At 100 or 50 mg L−1, there are other thioether-containing compounds showing higher activities against R. solanacearum than that of BT (38%, 17%), including R groups are 3-methyl-4-(2,2,2-trifluoroethoxy)pyridine (E24, 50%, 28%), 2-trifluoromethylbenzyl (E18, 49%, 33%), 3-chloro-2-fluorobenzyl (E7, 48%, 45%), 2-fluoro-5-trifluoromethylbenzyl (E2, 44%, 30%), 3,5-difluorobenzyl (E23, 40%, 32%), 2-bromo-4-fluorobenzyl (E14, 40%) and 2-methylbenzyl (E17, 40%, 24%). Sulfone-containing compounds F4 (43%) and F5 (48%) could also show higher activities than that of BT against R. solanacearum. Among the sulfoxide-containing compounds, compounds G14 (43%) and G15 (42%) showed higher activities against R. solanacearum than that of BT. Their R groups are 3-methyl-4-(2,2,2-trifluoroethoxy)pyridine and 3,4,4-trifluorobut-3-en-1-yl, respectively. Sulfone-containing compound F4 (43%) and sulfoxide-containing compounds G6 (36%), G10 (37%), G15 (42%), G16 (25%) showed higher activities against R. solanacearum than that of corresponding thioether-containing compounds E4 (30%), E16 (30%), E20 (5%), E25 (3%) and E26 (10%). But the rest of sulfone-containing compounds or sulfoxide-containing compounds exhibited much lower activities than that of corresponding thioether-containing compounds, which is totally different with the activities against Xoo. Particularly, when R is 2,3-dichlorobenzyl, thioether-containing compound E6 could show the highest activity (67%), which is much higher than that of TC and BT, but the corresponding oxidized sulfone-containing compound F6 owned no activity (1%) against R. solanacearum. In addition, thioether-containing compound E13 also exhibited much higher activity (63%) against R. solanacearum than that of both TC and BT, however, sulfoxide-containing compound G3, the oxidized product of E13, are almost no activity against R. solanacearum.| Compounds | Activitya (%) | Compounds | Activitya (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Xoo | R. solanacearum | Xoo | R. solanacearum | ||||||
| 100 mg L−1 | 50 mg L−1 | 100 mg L−1 | 50 mg L−1 | 100 mg L−1 | 50 mg L−1 | 100 mg L−1 | 50 mg L−1 | ||
| a The antibacterial activities are mean of three independent experiments. | |||||||||
| E1 | 40 ± 1.2 | 18 ± 2.4 | 57 ± 0.3 | 54 ± 0.8 | F1 | 41 ± 1.9 | 29 ± 1.4 | 30 ± 1.0 | 25 ± 4.1 |
| E2 | 26 ± 0.3 | 16 ± 3.0 | 44 ± 3.0 | 30 ± 1.3 | F2 | 33 ± 1.6 | 28 ± 2.3 | 10 ± 1.1 | — |
| E3 | 9 ± 2.7 | — | 53 ± 2.5 | 44 ± 3.3 | F3 | 47 ± 3.5 | 31 ± 0.4 | 9 ± 1.8 | — |
| E4 | 13 ± 0.5 | 2 ± 1.2 | 30 ± 1.9 | 10 ± 2.3 | F4 | 32 ± 2.8 | 22 ± 0.8 | 43 ± 1.8 | 18 ± 2.8 |
| E5 | 27 ± 1.5 | 21 ± 2.3 | 64 ± 4.4 | 50 ± 3.5 | F5 | 45 ± 3.8 | 31 ± 1.7 | 48 ± 4.8 | 45 ± 3.5 |
| E6 | 14 ± 0.8 | 10 ± 4.2 | 67 ± 2.2 | 52 ± 4.7 | F6 | 38 ± 2.2 | 24 ± 2.3 | 1 ± 0.1 | — |
| E7 | 7 ± 2.2 | — | 48 ± 1.6 | 45 ± 2.9 | F7 | 31 ± 0.3 | 21 ± 0.3 | 28 ± 2.6 | 14 ± 5.0 |
| E8 | 32 ± 0.5 | 16 ± 2.4 | 8 ± 5.0 | — | F8 | 19 ± 0.1 | 21 ± 1.4 | 21 ± 3.4 | 12 ± 0.3 |
| E9 | 45 ± 4.4 | 33 ± 1.7 | 50 ± 4.0 | 11 ± 3.4 | F9 | 47 ± 3.5 | 32 ± 3.1 | 32 ± 1.8 | 21 ± 1.1 |
| E10 | 29 ± 0.3 | 10 ± 1.6 | 62 ± 2.3 | 45 ± 3.6 | F10 | 53 ± 4.2 | 42 ± 4.8 | 36 ± 1.9 | 34 ± 3.3 |
| E11 | 16 ± 4.9 | 7 ± 2.3 | 54 ± 2.0 | 27 ± 4.5 | G1 | 28 ± 1.5 | 29 ± 2.0 | 2 ± 1.3 | — |
| E12 | 14 ± 4.3 | 4 ± 2.4 | 50 ± 3.5 | 40 ± 1.2 | G2 | 29 ± 1.6 | 20 ± 2.8 | 15 ± 2.3 | 2 ± 0.3 |
| E13 | 39 ± 2.6 | 32 ± 4.8 | 63 ± 3.3 | 56 ± 2.0 | G3 | 35 ± 1.2 | 17 ± 2.4 | 14 ± 1.7 | 8 ± 0.3 |
| E14 | 35 ± 4.7 | 30 ± 1.0 | 40 ± 2.2 | 14 ± 4.0 | G4 | 35 ± 3.0 | 24 ± 2.6 | 28 ± 2.0 | 21 ± 1.5 |
| E15 | 41 ± 2.3 | 35 ± 4.5 | 7 ± 3.1 | — | G5 | 48 ± 2.0 | 32 ± 1.0 | 19 ± 2.0 | 14 ± 3.5 |
| E16 | 43 ± 2.7 | 33 ± 0.4 | 30 ± 2.1 | 14 ± 3.0 | G6 | 46 ± 2.2 | 30 ± 0.9 | 36 ± 4.3 | 11 ± 3.5 |
| E17 | 4 ± 1.5 | — | 40 ± 1.1 | 24 ± 0.7 | G7 | 36 ± 2.3 | 26 ± 0.2 | 25 ± 3.1 | 18 ± 3.2 |
| E18 | 23 ± 1.6 | 3 ± 0.9 | 49 ± 3.4 | 33 ± 4.4 | G8 | 44 ± 4.9 | 24 ± 5.0 | 26 ± 0.4 | 23 ± 1.5 |
| E19 | 44 ± 1.7 | 26 ± 0.5 | 34 ± 3.8 | 27 ± 5.0 | G9 | 37 ± 4.4 | 20 ± 3.5 | 34 ± 0.3 | 29 ± 1.2 |
| E20 | 43 ± 1.7 | 35 ± 3.6 | 5 ± 1.0 | — | G10 | 33 ± 2.2 | 23 ± 3.8 | 37 ± 5.0 | 20 ± 1.1 |
| E21 | 35 ± 2.7 | 21 ± 2.1 | 23 ± 3.9 | 9 ± 0.8 | G11 | 36 ± 1.4 | 22 ± 4.8 | 2 ± 0.2 | — |
| E22 | 42 ± 4.2 | 25 ± 4.1 | 34 ± 3.9 | 18 ± 0.5 | G12 | 18 ± 1.1 | 10 ± 1.4 | 3 ± 1.8 | — |
| E23 | 42 ± 1.6 | 21 ± 2.8 | 40 ± 3.2 | 32 ± 2.3 | G13 | 39 ± 4.1 | 29 ± 3.9 | 11 ± 0.7 | 7 ± 0.5 |
| E24 | 38 ± 4.0 | 31 ± 4.2 | 50 ± 0.3 | 28 ± 0.6 | G14 | 35 ± 3.9 | 17 ± 0.3 | 43 ± 5.0 | 19 ± 2.3 |
| E25 | 33 ± 2.6 | 23 ± 3.9 | 3 ± 0.9 | — | G15 | 36 ± 2.2 | 26 ± 0.8 | 42 ± 3.4 | 38 ± 2.4 |
| E26 | 28 ± 3.9 | 15 ± 2.0 | 10 ± 4.2 | — | G16 | 40 ± 2.9 | 35 ± 3.8 | 25 ± 2.3 | 23 ± 0.1 |
| Thiodiazole copper (TC) | 53 ± 0.8 | 39 ± 4.6 | 51 ± 3.0 | 40 ± 4.3 | Bismerthiazol (BT) | 51 ± 3.7 | 31 ± 1.8 | 38 ± 2.5 | 17 ± 1.5 |
2.4 The EC50 values of active title compounds against Xoo or R. solanacearum
The half-maximal effective concentration (EC50) values of sulfone-containing compound F10 against Xoo, and thioether-containing compounds E1, E3, E5, E6, E10, E11, and E13 against R. solanacearum were further evaluated. The results listed in Table 2 indicated the EC50 value against Xoo of sulfone-containing compound F10 is 83 mg L−1, which is much lower than that of TC (97 mg L−1) and BT (112 mg L−1). Furthermore, the EC50 values against R. solanacearum of thioether-containing compounds E6 and E13 are 41 mg L−1 and 40 mg L−1 respectively, which are twice lower than that of TC (87 mg L−1) and third lower than that of BT (124 mg L−1). Thioether-containing compounds E1, E3, E5, E10 and E11 had the EC50 values of 53, 75, 53, 78 and 73 mg L−1 respectively, which are also much lower than that of TC and BT.| Compounds | Xoo | Compounds | R. solanacearum | ||||
|---|---|---|---|---|---|---|---|
| Regression equation | R2 | EC50a (mg L−1) | Regression equation | R2 | EC50a (mg L−1) | ||
| a Each experiment of EC50 value is performed in triplicates. | |||||||
| F10 | y = 0.8557x + 3.3573 | 1.00 | 83 ± 0.1 | E1 | y = 0.7768x + 3.661 | 0.98 | 53 ± 0.3 |
| Thiodiazole copper (TC) | y = 0.996x + 3.065 | 0.99 | 97 ± 1.2 | E3 | y = 2.4159x + 0.4676 | 0.94 | 75 ± 0.5 |
| Bismerthiazol (BT) | y = 2.5861x − 0.0104 | 0.97 | 112 ± 1.2 | E5 | y = 1.4452x + 2.5102 | 1.00 | 53 ± 0.9 |
| E6 | y = 2.3726x + 1.167 | 1.00 | 41 ± 0.2 | ||||
| E10 | y = 1.34x + 2.4627 | 0.97 | 78 ± 0.2 | ||||
| E11 | y = 1.3523x + 2.4812 | 0.99 | 73 ± 1.2 | ||||
| E13 | y = 0.9336x + 3.507 | 0.99 | 40 ± 2.1 | ||||
| Thiodiazole copper (TC) | y = 1.6934x + 1.7154 | 0.94 | 87 ± 1.1 | ||||
| Bismerthiazol (BT) | y = 2.0182x + 0.7772 | 0.97 | 124 ± 1.3 | ||||
2.5 Insecticidal activity test
The insecticidal activities of synthesized compounds against P. xylostella are shown in Table 3. The chlorpyrifos and avermectin were used as positive controls. The results (Table 3) revealed that some compounds could show moderate insecticidal activities. Thioether-containing compound E3 where R is 4-bromo-2-fluorobenzyl showed the highest activity of 75% against P. xylostella, but the activity could sharply decrease after being oxidized into corresponding sulfone-containing compound F3 (10%). When R was changed to 4-trifluoromethylbenzyl (E11) or 3-methyl-4-(2,2,2-trifluoroethoxy)pyridine (E24), the activity could slightly decrease to 70%. Thioether-containing compounds E5 and E12 both showed the activities of 55% against P. xylostella, and their R groups are 3-fluorobenzyl and 2-chloro-4-fluorobenzyl, respectively. Thioether-containing compounds E1 (2-bromo-5-fluorobenzyl), E4 (4-fluorobenzyl), E7 (3-chloro-2-fluorobenzyl), E15 (3-bromobenzyl) all showed activities of 50%. The rest of thioether-containing compounds showed lower activities less than 50%. In addition, the insecticidal activities of sulfone-containing compounds could be sorted as follows: 3-chloro-2-fluorobenzyl (F7) > 2-fluoro-5-trifluoromethylbenzyl (F2) > 2-bromo-5-fluorobenzyl (F4) > 4-fluorobenzyl (F1) > 2,3-dichlorobenzyl (F6) = 3,4-difluorobenzyl (F8) = benzyl (F9) = 3,4-dimethoxypyridin (F10) > 4-bromo-2-fluorobenzyl (F3) > 3-fluorobenzyl (F5). Specially, sulfone-containing compounds F2, F7, F9 and F10 all showed higher activities than that of corresponding thioether-containing compounds E2, E7, E9 and E10, but the rest of sulfone-containing compounds all showed activities less than that of corresponding thioether-containing compounds. When R is 2-chloro-4-fluorobenzyl, sulfoxide-containing compound G2 showed activity of 75%. Sulfoxide-containing compounds G3, G6 and G7 showed the activities of 60%, 60% and 50% respectively, which are all higher than that of corresponding thioether-containing compounds E13, E16 and E17. The activities of the rest of sulfoxide-containing compounds are all less than 50%.| Compounds | Activitya (%) at 500 mg L−1 | Compounds | Activitya (%) at 500 mg L−1 |
|---|---|---|---|
| a The each insecticidal test were performed in triplicates. | |||
| E1 | 50 ± 0 | F1 | 35 ± 0 |
| E2 | 45 ± 2.9 | F2 | 50 ± 0 |
| E3 | 75 ± 0 | F3 | 10 ± 3.3 |
| E4 | 50 ± 0 | F4 | 40 ± 3.3 |
| E5 | 55 ± 0 | F5 | 10 ± 0 |
| E6 | 40 ± 2.9 | F6 | 30 ± 3.3 |
| E7 | 50 ± 3.3 | F7 | 60 ± 0 |
| E8 | 30 ± 0 | F8 | 30 ± 0 |
| E9 | 10 ± 5 | F9 | 30 ± 0 |
| E10 | 10 ± 0 | F10 | 30 ± 0 |
| E11 | 70 ± 0 | G1 | 30 ± 0 |
| E12 | 55 ± 3.3 | G2 | 75 ± 0 |
| E13 | 30 ± 2.9 | G3 | 60 ± 0 |
| E14 | 10 ± 2.9 | G4 | 40 ± 0 |
| E15 | 50 ± 0 | G5 | 30 ± 0 |
| E16 | 20 ± 0 | G6 | 60 ± 0 |
| E17 | 40 ± 0 | G7 | 50 ± 2.9 |
| E18 | 30 ± 0 | G8 | 20 ± 0 |
| E19 | 20 ± 0 | G9 | 20 ± 0 |
| E20 | 30 ± 5 | G10 | 10 ± 0 |
| E21 | 20 ± 3.3 | G11 | 30 ± 0 |
| E22 | 30 ± 0 | G12 | 10 ± 0 |
| E23 | 35 ± 0 | G13 | 20 ± 0 |
| E24 | 70 ± 0 | G14 | 10 ± 3.3 |
| E25 | 10 ± 0 | G15 | 40 ± 0 |
| E26 | 10 ± 0 | G16 | 30 ± 3.3 |
| Chlorpyrifos | 100 ± 0 | Avermectin | 100 ± 0 |
3 Conclusion
A series of sulfur-containing trifluoromethylpyridine amide derivatives has been designed and synthesized. Their antibacterial activities against Xanthomonas oryzae pv. oryzae (Xoo), Ralstonia solanacearum (R. solanacearum) and insecticidal activities against P. xylostella were evaluated. Notably, sulfone-containing compound F10 (53%, 42%) showing the highest activity against Xoo had the EC50 of 83 mg L−1, which is much lower than that of TC (97 mg L−1) and BT (112 mg L−1). Thioether-containing compounds E1 (57%, 54%), E3 (53%, 44%), E5 (64%, 50%), E6 (67%, 52%), E9 (50%, 11%), E10 (62%, 45%), E11 (54%, 27%), and E13 (53%, 44%) showed higher activities than that of TC (51%, 40%) showed excellent antibacterial activities against R. solanacearum with EC50 values ranging from 40–73 mg L−1, which are all much lower than that of TC (87 mg L−1) and BT (124 mg L−1). Generally, most of oxidized compounds could show higher activities against Xoo than that of corresponding thioether-containing compounds, but most of thioether-containing compounds contributed higher activities against R. solanacearum. Furthermore, compounds E3, E11, E24 and G2 showed also moderate insecticidal activities of 75%, 70%, 70% and 75%, respectively.4 Experimental section
4.1 Materials and methods
All reagents and solvents were purchased from Accela Chem-Bio Co., Ltd (Shanghai, China) and Innochem Co., Ltd (Beijing, China). Melting points of the synthesized compounds were measured using a XT-4 binocular microscope (Beijing Tech Instrument Co., China). Using CDCl3 as solvent, the spectra of 1H, 19F and 13C NMR of title compounds were recorded on AVANCE III HD 400M NMR (Bruker Corporation, Switzerland) spectrometer operating at room temperature. HR-MS was recorded on an Orbitrap LC-MS instrument (Q-Exative, Thermo Scientific™, and American). The course of the reactions was monitored by TLC.4.2 Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Along with similar method, thioether-containing compounds E2–E26 could be also obtained. The spectral data of E1–E26 are listed below, and the spectra are shown in the ESI data.†
3). Along with similar method, thioether-containing compounds E2–E26 could be also obtained. The spectral data of E1–E26 are listed below, and the spectra are shown in the ESI data.†
3-Chloro-N-(2-((4-fluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E1). Yield 83%; yellow solid; mp 96–97 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H, pyridine-H), 8.08 (s, 1H, pyridine-H), 7.99 (s, 1H, CO–NH), 7.30 (dd, J = 8.4, 5.4 Hz, 2H, Ar–H), 6.98 (t, J = 8.6 Hz, 2H, Ar–H), 3.75 (s, 2H, –CH2), 3.62 (q, J = 6.4 Hz, 2H, –CH2), 2.69 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.3, 161.9 (d, J = 245.8 Hz), 149.1, 142.8 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 133.7 (d, J = 3.2 Hz), 132.2, 130.4 (d, J = 8.1 Hz), 129.3 (q, J = 34.0 Hz), 122.2 (q, J = 273.4 Hz), 115.5 (d, J = 21.5 Hz), 38.4, 35.2, 30.9. 19F NMR (376 MHz, CDCl3) δ −62.52, −115.22. HRMS: [M + H]+ calcd for C16H14ClF4N2OS: 393.04460; found: 393.04370.
3-Chloro-N-(2-((2-fluoro-5-(trifluoromethyl)benzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E2). Yield 83%; yellow solid; mp 101–103 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H, pyridine-H), 8.08 (d, J = 1.3 Hz, 2H, pyridine-H, CO–NH), 7.69 (dd, J = 6.7, 2.0 Hz, 1H, Ar–H), 7.54–7.49 (m, 1H, Ar–H), 7.16 (t, J = 8.9 Hz, 1H, Ar–H), 3.85 (s, 2H, –CH2), 3.69 (q, J = 6.4 Hz, 2H, –CH2), 2.77 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.5 (dd, J = 252.5, 1.3 Hz), 162.3, 148.9 (d, J = 1.0 Hz), 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 132.2, 129.3 (dd, J = 102.0 Hz, J = 34.0 Hz), 128.6–128.0 (m), 127.0 (dd, J = 33.1, 3.6 Hz), 126.7 (d, J = 16.0 Hz), 126.4 (dt, J = 13.0, 3.7 Hz), 123.6 (q, J = 272.0 Hz), 122.2 (q, J = 273.4 Hz), 116.2 (d, J = 23.4 Hz), 38.3, 31.5, 28.5 (d, J = 2.7 Hz). 19F NMR (376 MHz, CDCl3) δ −61.97, −62.57, δ −112.57 (dd, J = 14.2, 7.8 Hz). HRMS: [M + H]+ calcd for C17H13ClF7N2OS: 461.03199; found: 461.03098.
N-(2-((4-Bromo-2-fluorobenzyl)thio)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (E3). Yield 77%; brown solid; mp 95–96 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.0 Hz, 1H, pyridine-H), 8.08 (d, J = 1.3 Hz, 1H, –CO–NH), 8.05 (s, 1H, pyridine-H), 7.30–7.12 (m, 3H, Ar–H), 3.75 (s, 2H, –CH2), 3.67 (q, J = 6.4 Hz, 2H, –CH2), 2.73 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.3, 160.6 (d, J = 251.4 Hz), 149.0, 142.9 (q, J = 3.7 Hz), 137.7 (q, J = 3.6 Hz), 132.2, 132.0 (d, J = 4.5 Hz), 129.3 (q, J = 34.0 Hz), 127.7 (d, J = 3.7 Hz), 124.7 (d, J = 14.9 Hz), 122.2 (d, J = 273.4 Hz), 121.2 (d, J = 9.5 Hz), 119.2 (d, J = 25.1 Hz), 38.4, 31.2, 28.4 (d, J = 2.6 Hz). 19F NMR (376 MHz, CDCl3) δ −62.52, −115.12. HRMS: [M + H]+ calcd for C16H13BrClF4N2OS: 470.95511; found: 470.95456.
N-(2-((2-Bromo-5-fluorobenzyl)thio)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (E4). Yield 87%; gray solid; mp 111–112 °C. 1H NMR (400 MHz, CDCl3) δ 8.77–8.68 (m, 1H, pyridine-H), 8.14–8.01 (m, 2H, pyridine-H, –CO–NH), 7.50 (dd, J = 8.8, 5.3 Hz, 1H, Ar–H), 7.20 (dd, J = 9.1, 3.0 Hz, 1H, Ar–H), 6.85 (td, J = 8.3, 3.0 Hz, 1H, Ar–H), 3.87 (s, 2H, –CH2), 3.69 (q, J = 6.4 Hz, 2H, –CH2), 2.78 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.3, 161.9 (d, J = 247.6 Hz), 149.0, 142.9 (q, J = 3.8 Hz), 139.6 (d, J = 7.3 Hz), 137.7 (q, J = 3.6 Hz), 134.2 (d, J = 8.0 Hz), 132.2, 129.3 (q, J = 34.0 Hz), 122.2 (d, J = 273.4 Hz), 118.5 (d, J = 3.3 Hz), 117.8 (d, J = 23.3 Hz), 116.1 (d, J = 22.4 Hz), 38.5, 36.1, 31.4. 19F NMR (376 MHz, CDCl3) δ −62.52, −114.19. HRMS: [M + H]+ calcd for C16H13BrClF4N2OS: 470.95511; found: 470.95468.
3-Chloro-N-(2-((3-fluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E5). Yield 66%; yellow solid; mp 103–104 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.08 (d, J = 1.3 Hz, 1H, pyridine-H), 8.02 (s, 1H, –CO–NH), 7.33–7.19 (m, 1H, Ar–H), 7.13–7.06 (m, 2H, Ar–H), 6.92 (td, J = 8.3, 2.0 Hz, 1H, Ar–H), 3.76 (s, 2H, –CH2), 3.63 (q, J = 6.3 Hz, 2H, –CH2), 2.71 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.9 (d, J = 246.4 Hz), 162.3, 149.0, 142.9 (q, J = 3.8 Hz), 140.6 (d, J = 7.2 Hz), 137.7 (q, J = 3.5 Hz), 132.2, 130.1 (d, J = 8.3 Hz), 129.3 (q, J = 33.9 Hz), 124.6 (d, J = 2.8 Hz), 122.2 (d, J = 273.4 Hz), 115.8 (d, J = 21.7 Hz), 114.2 (d, J = 21.1 Hz), 38.3, 35.5 (d, J = 1.8 Hz), 31.0. 19F NMR (376 MHz, CDCl3) δ −62.52, −112.81. HRMS: [M − H]− calcd for C16H12ClF4N2OS: 391.02895; found: 391.03027.
3-Chloro-N-(2-((2,3-dichlorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E6). Yield 80%; white solid; mp 130–132 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (dd, J = 1.8, 0.7 Hz, 1H, pyridine-H), 8.12–8.01 (m, 2H, pyridine-H, –CO–NH), 7.35 (dd, J = 8.0, 1.6 Hz, 1H, Ar–H), 7.31 (dd, J = 7.7, 1.6 Hz, 1H, Ar–H), 7.16 (t, J = 7.8 Hz, 1H, Ar–H), 3.92 (s, 2H, –CH2), 3.68 (q, J = 6.4 Hz, 2H, –CH2), 2.77 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.3, 149.0, 142.9 (q, J = 3.8 Hz), 138.2, 137.7 (q, J = 3.6 Hz), 133.6, 132.4, 132.2, 129.4, 129.3 (q, J = 34.0 Hz), 128.9, 127.2, 122.2 (d, J = 273.5 Hz), 38.6, 34.5, 31.4. 19F NMR (376 MHz, CDCl3) δ −62.51. HRMS: [M + H]+ calcd for C16H13Cl3F3N2OS: 442.97608; found: 442.97546.
3-Chloro-N-(2-((3-chloro-2-fluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E7). Yield 81%; gray solid; mp 97–98 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.07 (t, J = 3.1 Hz, 2H, pyridine-H, –CO–NH), 7.35–7.16 (m, 2H, Ar–H), 7.04 (td, J = 7.9, 1.1 Hz, 1H, Ar–H), 3.81 (d, J = 0.8 Hz, 2H, –CH2), 3.68 (q, J = 6.4 Hz, 2H, –CH2), 2.76 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.3, 157.5, 149.0, 142.9 (q, J = 3.9 Hz), 137.7 (q, J = 3.6 Hz), 132.2, 129.6, 129.3 (q, J = 34.0 Hz), 129.2 (d, J = 3.3 Hz), 127.3 (d, J = 14.8 Hz), 124.6 (d, J = 4.8 Hz), 122.2 (q, J = 273.3 Hz), 121.3 (d, J = 18.0 Hz), 38.4, 31.3, 29.0 (d, J = 2.8 Hz). 19F NMR (376 MHz, CDCl3) δ −62.52, −119.81. HRMS: [M − H]− calcd for C16H11Cl2F4N2OS: 424.98998; found: 424.99158.
3-Chloro-N-(2-((3,4-difluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E8). Yield 68%; white solid; mp 97–98 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H, pyridine-H), 8.09 (s, 1H, pyridine-H), 8.02 (s, 1H, –CO–NH), 7.29–7.17 (m, 1H, Ar–H), 7.14–7.01 (m, 2H, Ar–H), 3.73 (s, 2H, –CH2), 3.67–3.58 (m, 2H, –CH2), 2.70 (td, J = 6.5, 1.6 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.3, 150.3 (dd, J = 248.8, 12.9 Hz), 149.5 (dd, J = 247.9, 12.7 Hz), 148.9, 142.9 (q, J = 3.7 Hz), 137.8 (q, J = 3.6 Hz), 135.1 (dd, J = 5.2, 4.1 Hz), 132.2, 129.3 (q, J = 33.9 Hz), 124.8 (dd, J = 6.2, 3.6 Hz), 122.2 (q, J = 273.5 Hz), 117.7 (d, J = 17.4 Hz), 117.2 (d, J = 17.2 Hz), 38.3, 35.1, 30.9. 19F NMR (376 MHz, CDCl3) δ −62.54, δ −137.19 (d, J = 21.2 Hz), −139.64 (d, J = 21.2 Hz). HRMS: [M − H]− calcd for C16H11ClF5N2OS: 409.01953; found: 409.02078.
N-(2-(Benzylthio)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (E9). Yield 75%; brown solid; mp 109–110 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.0 Hz, 1H, pyridine-H), 8.07 (d, J = 1.3 Hz, 1H, pyridine-H), 8.01 (s, 1H, –CO–NH), 7.36–7.27 (m, 4H, Ar–H), 7.26–7.20 (m, 1H, Ar–H), 3.78 (s, 2H, –CH2), 3.62 (q, J = 6.3 Hz, 2H, –CH2), 2.70 (t, J = 6.5 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.3, 149.2, 142.9 (q, J = 3.8 Hz), 138.0, 137.7 (q, J = 3.6 Hz), 132.2, 129.2 (q, J = 34.1 Hz), 128.9, 128.6, 127.2, 122.2 (q, J = 273.5 Hz), 38.3, 35.9, 30.9. 19F NMR (376 MHz, CDCl3) δ −62.51. HRMS: [M − H]− calcd for C16H13ClF3N2OS: 373.03955; found: 373.03837.
3-Chloro-N-(2-(((3,4-dimethoxypyridin-2-yl)methyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E10). Yield 60%; gray solid; mp 76–77 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.0 Hz, 1H, pyridine-H), 8.45 (s, 1H, pyridine-H), 8.14 (d, J = 5.5 Hz, 1H, pyridine-H), 8.06 (d, J = 1.3 Hz, 1H, –CO–NH), 6.76 (d, J = 5.6 Hz, 1H, pyridine-H), 3.97–3.86 (m, 8H, –(OCH3)2, –CH2), 3.73 (dd, J = 12.3, 6.0 Hz, 2H, –CH2), 2.86 (t, J = 6.2 Hz, 2H, –CH2). 13C NMR (100 MHz, CDCl3) δ 162.5, 158.8, 152.7, 150.0, 145.4, 143.4, 142.9 (q, J = 3.8 Hz), 137.3 (q, J = 3.6 Hz), 131.9, 129.0 (q, J = 33.9 Hz), 122.2 (q, J = 273.4 Hz), 106.9, 61.1, 55.7, 39.1, 31.69, 31.4. 19F NMR (376 MHz, CDCl3) δ −62.50. HRMS: [M + H]+ calcd for C17H18ClF3N3O3S: 436.07040; found: 436.06998.
3-Chloro-5-(trifluoromethyl)-N-(2-((4-(trifluoromethyl)benzyl)thio)ethyl)picolinamide (E11). Yield 88%; yellow solid; mp 114–115 °C. 1H NMR (400 MHz, CDCl3) δ 8.75–8.70 (m, 1H, pyridine-H), 8.12–8.06 (m, 1H, pyridine-H), 8.03 (s, 1H, –CO–NH), 7.57 (d, J = 8.1 Hz, 2H, Ar–H), 7.47 (d, J = 8.1 Hz, 2H), 3.82 (s, 2H), 3.65 (q, J = 6.5 Hz, 2H), 2.69 (t, J = 6.6 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 148.9, 142.9 (q, J = 3.9 Hz), 142.2, 137.8 (q, J = 3.6 Hz), 132.2, 129.4 (q, J = 32.6 Hz), 129.3 (q, J = 34.0 Hz), 129.2, 125.6 (q, J = 3.8 Hz), 124.1 (q, J = 272.0 Hz), 122.1 (q, J = 273.4 Hz), 38.3, 35.4, 30.9. 19F NMR (376 MHz, CDCl3) δ −62.48, −62.54. HRMS: [M + H]+ calcd for C17H14ClF6N2OS: 443.04041; found: 443.04141.
3-Chloro-N-(2-((2-chloro-4-fluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E12). Yield 68%; gray solid; mp 90–91 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H), 8.08 (d, J = 0.4 Hz, 2H), 7.38 (dd, J = 8.5, 6.1 Hz, 1H), 7.18–7.06 (m, 1H), 6.95 (ddd, J = 8.2, 2.5, 1.2 Hz, 1H), 3.86 (s, 2H), 3.68 (q, J = 6.4 Hz, 2H), 2.75 (t, J = 6.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 161.6 (d, J = 249.7 Hz), 149.0, 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.5 Hz), 134.6 (d, J = 10.3 Hz), 132.2, 131.8, 131.7, 129.3 (q, J = 34.0 Hz), 122.2 (q, J = 273.4 Hz), 117.2 (d, J = 24.7 Hz), 114.2 (d, J = 21.1 Hz), 38.6, 32.8, 31.2. 19F NMR (376 MHz, CDCl3) δ −62.52, −112.69. HRMS: [M + H]+ calcd for C16H13Cl2F4N2OS: 427.00563; found: 427.00470.
3-Chloro-N-(2-((4-isopropylbenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E13). Yield 79%; yellow solid; mp 98–100 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H), 8.11–8.06 (m, 1H), 8.03 (s, 1H), 7.28–7.23 (m, 2H), 7.17 (d, J = 8.1 Hz, 2H), 3.75 (s, 2H), 3.64 (dd, J = 12.7, 6.3 Hz, 2H), 2.88 (dt, J = 13.9, 6.9 Hz, 1H), 2.70 (t, J = 6.5 Hz, 2H), 1.23 (d, J = 6.9 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 162.2, 149.2, 147.9, 142.8 (q, J = 3.8 Hz), 137.6 (q, J = 3.5 Hz), 135.1, 132.1, 129.2 (d, J = 34.0 Hz), 128.8, 126.7, 122.2 (q, J = 273.5 Hz), 38.3, 35.5, 33.8, 30.8, 24.0. 19F NMR (376 MHz, CDCl3) δ −62.52. HRMS: [M − H]− calcd for C19H19ClF3N2OS: 415.08532; found: 415.08630.
N-(2-((2-Bromo-4-fluorobenzyl)thio)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (E14). Yield 64%; yellow solid; mp 92–94 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H), 8.08 (d, J = 1.3 Hz, 1H), 8.05 (s, 1H), 7.39 (dd, J = 8.5, 5.9 Hz, 1H), 7.29 (dd, J = 8.2, 2.6 Hz, 1H), 6.99 (td, J = 8.3, 2.6 Hz, 1H), 3.87 (s, 2H), 3.68 (q, J = 6.4 Hz, 2H), 2.76 (t, J = 6.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 161.4 (d, J = 250.8 Hz), 149.1, 142.8 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 133.5 (d, J = 3.6 Hz), 132.2, 131.7 (d, J = 8.4 Hz), 129.3 (q, J = 34.0 Hz), 124.4 (d, J = 9.6 Hz), 122.2 (q, J = 273.4 Hz), 120.3 (d, J = 24.5 Hz), 114.8 (d, J = 21.1 Hz), 38.6, 35.5, 31.3. 19F NMR (376 MHz, CDCl3) δ −62.55, −112.79. HRMS: [M + H]+ calcd for C16H13BrClF4N2OS: 470.95511; found: 470.95468.
N-(2-((3-Bromobenzyl)thio)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (E15). Yield 82%; gray solid; mp 89–91 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 1.0 Hz, 1H), 8.07 (d, J = 1.4 Hz, 1H), 8.01 (s, 1H), 7.51 (t, J = 1.6 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.26 (t, J = 3.8 Hz, 1H), 7.17 (t, J = 7.8 Hz, 1H), 3.73 (s, 2H), 3.63 (q, J = 6.3 Hz, 2H), 2.71 (t, J = 6.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 149.1, 142.9 (q, J = 3.8 Hz), 140.4, 137.7 (q, J = 3.5 Hz), 132.2, 131.9, 130.3, 130.1, 129.3 (q, J = 33.9 Hz), 127.5, 122.7, 122.2 (q, J = 273.4 Hz), 38.4, 35.5, 31.1. 19F NMR (376 MHz, CDCl3) δ −62.54. HRMS: [M + H]+ calcd for C16H14BrClF3N2OS: 452.96454; found: 452.96402.
3-Chloro-N-(2-((2,6-difluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E16). Yield 72%; white solid; mp 112–113 °C. 1H NMR (400 MHz, CDCl3) δ 8.76–8.70 (m, 1H), 8.07 (dd, J = 1.2, 0.6 Hz, 2H), 7.25–7.16 (m, 1H), 6.93–6.85 (m, 2H), 3.82 (s, 2H), 3.71 (q, J = 6.2 Hz, 2H), 2.80 (t, J = 6.4 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 161.1 (dd, J = 248.7, 7.9 Hz), 162.2, 149.1, 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.4 Hz), 132.2, 129.2 (q, J = 34.0 Hz), 128.8 (t, J = 10.3 Hz), 122.2 (q, J = 273.4 Hz), 115.0 (t, J = 19.2 Hz), 111.4 (q, J = 12.6 Hz), 38.3, 31.6, 22.3. 19F NMR (376 MHz, CDCl3) δ −62.52, −115.00. HRMS: [M + H]+ calcd for C16H13ClF5N2OS: 411.03518; found: 411.03448.
3-Chloro-N-(2-((2-methylbenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E17). Yield 82%; gray solid; mp 84–85 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.0 Hz, 1H), 8.07 (d, J = 1.3 Hz, 1H), 8.02 (s, 1H), 7.25–7.19 (m, 1H), 7.16–7.11 (m, 3H), 3.78 (s, 2H), 3.65 (q, J = 6.3 Hz, 2H), 2.74 (t, J = 6.5 Hz, 2H), 2.41 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 162.2, 149.1, 142.9 (q, J = 3.9 Hz), 137.7 (q, J = 3.6 Hz), 136.7, 135.6, 132.2, 130.9, 129.7, 129.2 (d, J = 33.9 Hz), 127.5, 126.0, 122.2 (d, J = 273.3 Hz), 38.6, 34.2, 31.3, 19.2. 19F NMR (376 MHz, CDCl3) δ −62.50. HRMS: [M + H]+ calcd for C17H17ClF3N2OS: 389.06967; found: 389.06924.
3-Chloro-5-(trifluoromethyl)-N-(2-((2-(trifluoromethyl)benzyl)thio)ethyl)picolinamide (E18). Yield 60%; yellow solid; mp 77–78 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 0.8 Hz, 1H), 8.08 (dd, J = 1.3, 0.5 Hz, 2H), 7.64 (t, J = 8.4 Hz, 2H), 7.52 (t, J = 7.5 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 3.96 (s, 2H), 3.66 (q, J = 6.4 Hz, 2H), 2.79 (t, J = 6.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 149.0, 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 136.8 (d, J = 1.4 Hz), 132.2, 132.1, 131.5, 129.3 (q, J = 33.9 Hz), 128.5 (q, J = 29.9 Hz), 127.3, 126.2 (q, J = 5.6 Hz), 124.3 (d, J = 274.0 Hz), 122.2 (d, J = 273.5 Hz), 38.5, 32.5 (d, J = 2.0 Hz), 31.9. 19F NMR (376 MHz, CDCl3) δ −59.05, −62.53. HRMS: [M − H]− calcd for C17H12ClF6N2OS: 441.02704; found: 441.02576.
3-Chloro-N-(2-((2-chlorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E19). Yield 43%; brown solid; mp 77–78 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H), 8.07 (d, J = 1.3 Hz, 2H), 7.39 (dd, J = 7.3, 1.9 Hz, 1H), 7.36 (dd, J = 7.6, 1.6 Hz, 1H), 7.25–7.16 (m, 2H), 3.90 (s, 2H), 3.68 (q, J = 6.3 Hz, 2H), 2.76 (t, J = 6.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 149.1, 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 135.8, 134.0, 132.2, 130.9, 129.9, 129.2 (q, J = 34.1 Hz), 128.7, 127.0, 122.2 (q, J = 273.5 Hz), 38.6, 33.5, 31.3. 19F NMR (376 MHz, CDCl3) δ −62.51. HRMS: [M + H]+ calcd for C16H14Cl2F3N2OS: 409.01505; found: 409.01443.
N-(2-((4-(tert-Butyl)benzyl)thio)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (E20). Yield 84%; gray solid; mp 111–113 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 1.1 Hz, 1H), 8.08 (d, J = 1.3 Hz, 1H), 8.04 (s, 1H), 7.37–7.31 (m, 2H), 7.30–7.18 (m, 2H), 3.75 (s, 2H), 3.64 (q, J = 6.3 Hz, 2H), 2.70 (t, J = 6.5 Hz, 2H), 1.30 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 162.3, 150.2, 149.2, 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.5 Hz), 134.7, 132.2, 129.2 (q, J = 33.9 Hz), 128.6, 125.6, 122.2 (d, J = 273.4 Hz), 38.3, 35.4, 34.5, 31.3, 30.9. 19F NMR (376 MHz, CDCl3) δ −62.51. HRMS: [M + H]+ calcd for C20H23ClF3N2OS: 431.11662; found: 431.11563.
3-Chloro-N-(2-(((6-chloropyridin-3-yl)methyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E21). Yield 48%; brown solid; mp 88–89 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H), 8.34 (d, J = 2.2 Hz, 1H), 8.09 (s, 1H), 8.05 (s, 1H), 7.70 (dd, J = 8.2, 2.4 Hz, 1H), 7.29 (d, J = 8.2 Hz, 1H), 3.76 (s, 2H), 3.66 (q, J = 6.5 Hz, 2H), 2.70 (t, J = 6.6 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.4, 150.3, 149.7, 148.8, 142.9 (q, J = 3.8 Hz), 139.3, 137.8 (q, J = 3.6 Hz), 132.9, 132.3, 129.4 (q, J = 34.0 Hz), 124.3, 122.1 (q, J = 273.4 Hz), 38.4, 32.3, 31.0. 19F NMR (376 MHz, CDCl3) δ −62.52. HRMS: [M − H]− calcd for C15H11Cl2F3N3OS: 407.99465; found: 407.99591.
3-Chloro-N-(2-((4-cyanobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E22). Yield 83%; gray solid; mp 87–89 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.0 Hz, 1H), 8.12–8.07 (m, 1H), 8.03 (s, 1H), 7.61 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.2 Hz, 2H), 3.81 (s, 2H), 3.64 (q, J = 6.5 Hz, 2H), 2.68 (t, J = 6.7 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 148.9, 143.7, 142.9 (q, J = 3.8 Hz), 137.8 (q, J = 3.6 Hz), 132.4, 132.3, 129.7, 129.4 (q, J = 33.3 Hz), 122.1 (d, J = 273.4 Hz), 118.7, 111.1, 38.4, 35.6, 31.0. 19F NMR (376 MHz, CDCl3) δ −62.51. HRMS: [M − H]− calcd for C17H12ClF3N3OS: 398.03362; found: 398.03470.
3-Chloro-N-(2-((3,5-difluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E23). Yield 83%; white solid; mp 94–95 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 1.1 Hz, 1H), 8.13–8.07 (m, 1H), 8.03 (s, 1H), 6.99–6.82 (m, 2H), 6.68 (tt, J = 8.9, 2.3 Hz, 1H), 3.74 (s, 2H), 3.64 (q, J = 6.4 Hz, 2H), 2.72 (t, J = 6.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 163.0 (dd, J = 249.0, 12.8 Hz), 162.3, 148.9, 142.9 (q, J = 3.8 Hz), 142.1 (t, J = 9.0 Hz), 137.8 (q, J = 3.6 Hz), 132.3, 129.3 (q, J = 33.9 Hz), 122.2 (q, J = 273.5 Hz), 111.8 (q, J = 11.7 Hz), 102.8 (t, J = 25.3 Hz), 38.3, 35.5, 31.1. 19F NMR (376 MHz, CDCl3) δ −62.53, −109.51. HRMS: [M − H]− calcd for C16H11ClF5N2OS: 409.01953; found: 409.02075.
3-Chloro-N-(2-(((3-methyl-4-(trifluoromethoxy)pyridin-2-yl)methyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E24). Yield 77%; gray solid; mp 124–125 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H), 8.40 (s, 1H), 8.27 (d, J = 5.7 Hz, 1H), 8.06 (d, J = 1.1 Hz, 1H), 6.63 (d, J = 5.7 Hz, 1H), 4.39 (q, J = 7.9 Hz, 2H), 3.93 (s, 2H), 3.70 (dd, J = 12.4, 6.0 Hz, 2H), 2.82 (t, J = 6.3 Hz, 2H), 2.29 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 162.5, 161.8, 158.1, 149.8, 147.5, 142.9 (q, J = 3.8 Hz), 137.4 (q, J = 3.7 Hz), 131.9, 129.0 (q, J = 33.8 Hz), 123.0 (q, J = 277.8 Hz), 122.2 (q, J = 273.3 Hz), 121.1, 105.4, 65.4 (q, J = 36.3 Hz), 39.2, 35.4, 31.1, 10.6. 19F NMR (376 MHz, CDCl3) δ −62.53, −73.85. HRMS: [M + H]+ calcd for C18H17ClF6N3O2S: 488.06287; found: 488.06143.
3-Chloro-N-(2-((3,4,4-trifluorobut-3-en-1-yl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E25). Yield 84%; brown solid; mp 69–70 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 1.0 Hz, 1H), 8.11 (s, 1H), 8.08 (d, J = 1.4 Hz, 1H), 3.68 (q, J = 6.5 Hz, 2H), 2.83 (t, J = 5.4 Hz, 2H), 2.79 (t, J = 6.0 Hz, 2H), 2.60 (dddd, J = 11.1, 7.1, 5.3, 3.2 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 153.6 (ddd, J = 287.1, 273.3, 46.3 Hz), 148.9, 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 132.2, 129.3 (q, J = 34.0 Hz), 127.2 (ddd, J = 69.7, 56.0, 16.2 Hz), 122.2 (q, J = 273.4 Hz), 38.7, 31.6, 27.6, 26.4 (dd, J = 21.9, 2.6 Hz). 19F NMR (376 MHz, CDCl3) δ −62.55, −103.74 (dd, J = 85.2, 32.5 Hz), −123.06 (dd, J = 114.4, 85.2 Hz), −175.59 (dd, J = 114.2, 32.4 Hz). HRMS: [M + H]+ calcd for C12H8ClF4N2OS: 393.04372; found: 393.04463.
3-Chloro-N-(2-((2,5-difluorobenzyl)thio)ethyl)-5-(trifluoromethyl)picolinamide (E26). Yield 62%; white solid; mp 111–112 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 1.1 Hz, 1H), 8.08 (d, J = 1.3 Hz, 1H), 7.12 (ddd, J = 8.8, 5.8, 3.1 Hz, 1H), 7.00 (td, J = 9.0, 4.5 Hz, 1H), 6.94–6.86 (m, 1H), 3.77 (s, 2H), 3.67 (q, J = 6.3 Hz, 2H), 2.76 (t, J = 6.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.3, 158.6 (dd, J = 242.8, 2.4 Hz), 156.8 (dd, J = 242.2, 2.5 Hz), 149.0, 142.9 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 132.2, 129.3 (q, J = 34.0 Hz), 127.1 (dd, J = 17.4, 7.6 Hz), 122.2 (q, J = 273.3 Hz), 117.2 (dd, J = 24.4, 4.1 Hz), 116.6 (dd, J = 24.9, 8.7 Hz), 115.4 (dd, J = 22.8, 7.3 Hz), 38.3, 31.3, 28.6. 19F NMR (376 MHz, CDCl3) δ −62.53, δ −118.44 (d, J = 17.8 Hz), −124.24 (d, J = 17.8 Hz). HRMS: [M − H]− calcd for C16H11ClF5N2OS: 409.01953; found: 409.02078.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to obtain pure sulfone-containing compound F1. The sulfone-containing compounds F2–F10 could be also synthesized with similar method. The data of F1–F10 are listed below, and the spectra are shown in ESI data.†
3) to obtain pure sulfone-containing compound F1. The sulfone-containing compounds F2–F10 could be also synthesized with similar method. The data of F1–F10 are listed below, and the spectra are shown in ESI data.†
3-Chloro-N-(2-((4-fluorobenzyl)sulfonyl)ethyl)-5-(trifluoromethyl)picolinamide (F1). Yield 41%; white solid; mp 132–133 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.1 Hz, 1H, pyridine-H), 8.40 (t, J = 5.3 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.4 Hz, 1H, pyridine-H), 7.33–7.23 (m, 2H, Ar–H), 7.15–7.00 (m, 2H, Ar–H), 4.03 (q, J = 13.2 Hz, 2H, –CH2–), 3.96 (dd, J = 12.1, 6.1 Hz, 2H, –CH2–), 3.11–2.77 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.9 (d, J = 248.2 Hz), 162.8, 148.7, 143.0 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz), 132.1, 131.9 (d, J = 8.3 Hz), 129.4 (q, J = 34.1 Hz), 125.2 (d, J = 3.3 Hz), 122.1 (q, J = 273.3 Hz), 116.1 (d, J = 21.7 Hz), 57.4, 49.5, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.57, −112.80. HRMS: calculated for C16H14O3N2ClF4S [M + H]+: 435.03443; found: 425.03378.
3-Chloro-N-(2-((2-fluoro-5-(trifluoromethyl)benzyl)sulfonyl)ethyl)-5-(trifluoromethyl)picolinamide (F2). Yield 50%; white solid; mp 150–151 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 0.9 Hz, 1H, pyridine-H), 8.37 (s, 1H, –CO–NH–), 8.07 (d, J = 1.4 Hz, 1H, pyridine-H), 7.71–7.60 (m, 2H, Ar–H), 7.26 (dd, J = 11.5, 6.1 Hz, 1H, Ar–H), 4.14 (dd, J = 38.7, 13.2 Hz, 2H, –CH2–), 3.99 (q, J = 6.1 Hz, 2H, –CH2–), 3.33–2.55 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 162.8 (d, J = 253.8 Hz), 148.6, 143.0 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 132.2, 129.9 (q, J = 8.0 Hz), 129.4 (q, J = 34.1 Hz), 128.0 (d, J = 9.4 Hz), 127.4 (q, J = 33.4 Hz), 123.4 (q, J = 272.2 Hz), 122.1 (d, J = 273.5 Hz), 118.3 (d, J = 16.4 Hz), 116.5 (d, J = 23.2 Hz), 50.8, 50.3, 34.3. 19F NMR (376 MHz, CDCl3) δ −62.03, −62.60, −110.62. HRMS: calculated for C16H14O3N2ClF4S [M + H]+: 477.02690; found: 477.02563.
N-(2-((4-Bromo-2-fluorobenzyl)sulfonyl)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (F3). Yield 47%; yellow solid; mp 124–125 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.0 Hz, 1H, pyridine-H), 8.38 (t, J = 5.2 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.2 Hz, 1H, pyridine-H), 7.32 (d, J = 8.8 Hz, 2H, Ar–H), 7.25 (dd, J = 14.0, 6.0 Hz, 1H, Ar–H), 4.06 (dd, J = 36.8, 13.4 Hz, 2H, –CH2–), 3.96 (dt, J = 7.1, 3.4 Hz, 2H, –CH2–), 3.15–2.79 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 160.7 (d, J = 252.3 Hz), 148.7, 143.0 (q, J = 3.8 Hz), 137.6 (q, J = 3.5 Hz), 133.4 (d, J = 3.9 Hz), 132.1, 129.4 (q, J = 34.1 Hz), 128.1 (d, J = 3.7 Hz), 123.2, 122.1 (q, J = 273.5 Hz), 119.5 (d, J = 24.9 Hz), 116.0 (d, J = 15.3 Hz), 50.6, 50.0, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.57, −113.50. HRMS: calculated for C16H13O3N2BrClF4S [M + H]+: 502.94494; found: 502.94443.
N-(2-((2-Bromo-5-fluorobenzyl)sulfonyl)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (F4). Yield 44%; white solid; mp 146–147 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.41 (t, J = 5.3 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.3 Hz, 1H, pyridine-H), 7.58 (dd, J = 8.8, 5.2 Hz, 1H, Ar–H), 7.17 (dd, J = 8.7, 3.0 Hz, 1H, Ar–H), 6.97 (ddd, J = 8.8, 7.9, 3.0 Hz, 1H, Ar–H), 4.22 (dd, J = 48.0, 13.0 Hz, 2H, –CH2–), 4.05–3.95 (m, 2H, –CH2–), 3.25–2.90 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 161.7 (d, J = 249.1 Hz), 148.8, 143.1 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz) 134.5 (d, J = 8.1 Hz), 132.1, 131.8 (d, J = 8.0 Hz), 129.4 (q, J = 34.0 Hz), 122.1 (q, J = 273.4 Hz), 119.5 (d, J = 23.5 Hz), 119.2 (d, J = 3.5 Hz), 117.6 (d, J = 22.4 Hz), 58.3, 50.1, 34.5. 19F NMR (376 MHz, CDCl3) δ −62.57, −113.05. HRMS: calculated for C16H13O3N2BrClF4S [M + H]+: 502.94494; found: 502.94223.
3-Chloro-N-(2-((3-fluorobenzyl)sulfonyl)ethyl)-5-(trifluoromethyl)picolinamide (F5). Yield 50%; yellow solid; mp 97–98 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.41 (t, J = 5.3 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.3 Hz, 1H, pyridine-H), 7.41–7.30 (m, 1H, Ar–H), 7.16–6.94 (m, 3H, Ar–H), 4.10–4.00 (m, 2H, –CH2–), 4.00–3.93 (m, 2H, –CH2–), 3.13–2.79 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.9 (d, J = 247.8 Hz), 162.8, 148.8, 143.1 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz), 132.1, 131.7 (d, J = 7.7 Hz), 130.6 (d, J = 8.3 Hz), 129.4 (q, J = 34.0 Hz), 125.9 (d, J = 3.0 Hz), 122.1 (q, J = 273.4 Hz), 117.1 (d, J = 22.0 Hz), 115.7 (d, J = 21.0 Hz), 57.8, 49.7, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.57, −111.70. HRMS: calculated for C16H14O3N2ClF4S [M + H]+: 425.03443; found: 425.03293.
3-Chloro-N-(2-((2,3-dichlorobenzyl)sulfonyl)ethyl)-5-(trifluoromethyl)picolinamide (F6). Yield 44%; faint yellow solid; mp 161–162 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.41 (t, J = 5.5 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.3 Hz, 1H, pyridine-H), 7.48 (dd, J = 8.0, 1.6 Hz, 1H, Ar–H), 7.32 (dd, J = 7.7, 1.6 Hz, 1H, Ar–H), 7.23 (t, J = 7.8 Hz, 1H, Ar–H), 4.27 (dd, J = 39.0, 12.9 Hz, 2H, –CH2–), 4.01 (dd, J = 12.1, 6.1 Hz, 2H, –CH2–), 3.23–2.89 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 148.7, 143.1 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz), 134.0, 133.0, 132.1, 130.9, 130.6, 130.3, 129.4 (q, J = 34.0 Hz), 127.7, 122.1 (q, J = 273.5 Hz), 57.0, 50.2, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C16H13O3N2Cl3F4S [M + H]+: 474.96591; found: 474.96579.
3-Chloro-N-(2-((3-chloro-2-fluorobenzyl)sulfonyl)ethyl)-5-(trifluoromethyl)picolinamide (F7). Yield 40%; pale yellow solid; mp 145–146 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H), 8.39 (t, J = 5.5 Hz, 1H), 8.07 (d, J = 1.3 Hz, 1H), 7.45–7.36 (m, 1H), 7.30–7.24 (m, 1H), 7.12 (td, J = 7.9, 1.0 Hz, 1H), 4.13 (ddd, J = 30.9, 13.2, 1.1 Hz, 2H), 4.02–3.94 (m, 2H), 3.19–2.83 (m, 2H). 19F NMR (376 MHz, CDCl3) δ −62.56, −117.99. 13C NMR (100 MHz, CDCl3) δ 162.8, 156.5 (d, J = 249.8 Hz), 148.7, 143.0 (q, J = 3.9 Hz), 137.6 (q, J = 3.5 Hz), 132.1, 131.2, 130.6 (d, J = 2.7 Hz), 129.4 (q, J = 34.0 Hz), 125.1 (d, J = 4.8 Hz), 122.1 (q, J = 273.4 Hz), 121.7 (d, J = 17.9 Hz), 118.7 (d, J = 15.2 Hz), 51.2, 50.1, 34.4. HRMS: calculated for C16H13O3N2Cl2F4S [M + H]+: 458.99546; found: 458.99557.
3-Chloro-N-(2-((3,4-difluorobenzyl)sulfonyl)ethyl)-5-(trifluoromethyl)picolinamide (F8). Yield 84%; white solid; mp 163–164 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 1.1 Hz, 1H, pyridine-H), 8.32 (t, J = 5.5 Hz, 1H, –CO–NH–), 8.09 (d, J = 1.3 Hz, 1H, pyridine-H), 7.35–7.27 (m, 1H, Ar–H), 7.25–7.11 (m, 2H, Ar–H), 4.26 (s, 2H, –CH2–), 3.96 (dd, J = 12.2, 6.2 Hz, 2H, –CH2–), 3.44–3.07 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.6, 151.1 (dd, J = 248.7, 9.4 Hz), 150.5 (dd, J = 257.0, 19.3 Hz), 148.2, 143.1 (q, J = 3.8 Hz), 137.8 (q, J = 3.5 Hz), 132.3, 129.6 (q, J = 34.1 Hz), 127.1 (dd, J = 6.6, 3.8 Hz), 124.0 (dd, J = 6.1, 4.1 Hz), 122.1 (d, J = 273.5 Hz), 120.0 (d, J = 18.0 Hz), 118.1 (d, J = 17.5 Hz), 59.4, 50.8, 33.1. 19F NMR (376 MHz, CDCl3) δ −62.59, −135.47 (d, 3JF–F = 21.2 Hz), −135.85 (d, 3J F–F = 21.2 Hz). HRMS: calculated for C16H13O3N3ClF5S [M + H]+: 443.02501; found: 443.02417.
N-(2-(Benzylsulfonyl)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (F9). Yield 39%; soil white solid; mp 137–138 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.1 Hz, 1H, pyridine-H), 8.41 (t, J = 4.8 Hz, 1H, –CO–NH–), 8.06 (d, J = 1.3 Hz, 1H, pyridine-H), 7.41–7.33 (m, 3H, Ar–H), 7.30 (dt, J = 5.3, 4.3 Hz, 2H, Ar–H), 4.13–4.03 (m, 2H, –CH2–), 3.96 (hd, J = 9.2, 6.1 Hz, 2H, –CH2–), 3.08–2.76 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.7, 148.9, 143.1 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz), 132.1, 130.1, 129.4 (q, J = 23.9 Hz), 129.3, 129.1, 128.6, 122.1 (q, J = 273.4 Hz), 58.5, 49.3, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C16H15O3N2ClF3S [M + H]+: 407.04385; found: 407.04343.
3-Chloro-N-(2-(((3,4-dimethoxypyridin-2-yl)methyl)sulfonyl)ethyl)-5-(trifluoromethyl)picolinamide (F10). Yield 35%; pale yellow solid; mp 113–114 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.0 Hz, 1H, pyridine-H), 8.63 (t, J = 5.2 Hz, 1H, –CO–NH–), 8.21 (d, J = 5.5 Hz, 1H, pyridine-H), 8.05 (d, J = 1.3 Hz, 1H, pyridine-H), 6.82 (d, J = 5.5 Hz, 1H, pyridine-H), 4.37 (dd, J = 63.7, 12.4 Hz, 2H, –CH2–), 4.03 (dt, J = 8.4, 3.9 Hz, 2H, –CH2–), 3.92 (d, J = 3.4 Hz, 6H, –CH3), 3.28–3.04 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.69, 158.83, 149.33, 146.07, 145.09, 144.29, 143.01 (q, J = 3.8 Hz), 137.40 (q, J = 3.5 Hz), 131.90, 129.13 (q, J = 34.0 Hz), 122.16 (q, J = 273.4 Hz), 107.74, 61.50, 55.79, 54.66, 49.85, 34.33. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C17H18O5N3ClF3S [M + H]+: 468.06023; found: 468.06030.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Other sulfoxide-containing compounds G2–G16 could be also synthesized with the similar method. The confirming data are listed below, and the spectra are listed in ESI data.†
3). Other sulfoxide-containing compounds G2–G16 could be also synthesized with the similar method. The confirming data are listed below, and the spectra are listed in ESI data.†
3-Chloro-5-(trifluoromethyl)-N-(2-((4-(trifluoromethyl)benzyl) sulfinyl)ethyl)picolinamide (G1). Yield 54%; white solid; mp 184–185 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H, pyridine-H), 8.37 (br, 1H, –CO–NH–), 8.07 (s, 1H, pyridine-H), 7.65 (d, J = 8.0 Hz, 2H, Ar–H), 7.45 (d, J = 7.9 Hz, 2H, Ar–H), 4.10 (dd, J = 37.2, 13.0 Hz, 2H, –CH2–), 3.96 (dt, J = 10.8, 5.6 Hz, 2H, –CH2–), 3.0 (dtd, J = 18.8, 12.8, 5.9 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.83, 148.61, 143.03 (q, J = 3.8 Hz), 137.7 (q, J = 3.6 Hz), 133.5, 132.2, 130.8 (q, J = 32.7 Hz), 130.6, 129.5 (q, J = 34.0 Hz), 126.0 (q, J = 3.7 Hz), 123.9 (q, J = 272.3 Hz), 122.1 (q, J = 273.5 Hz), 57.6, 50.0, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.59, −62.76. HRMS: calculated for C17H14O2N2ClF6S [M + H]+: 459.03632; found: 459.03659.
3-Chloro-N-(2-((2-chloro-4-fluorobenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G2). Yield 60%; soil white solid; mp 165–166 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.38 (t, J = 5.2 Hz, 1H, –CO–NH–), 8.12–8.02 (m, 1H, pyridine-H), 7.46–7.37 (m, 1H, Ar–H), 7.32–7.23 (m, 1H, Ar–H), 7.12 (td, J = 8.0, 0.8 Hz, 1H, Ar–H), 4.14 (dt, J = 29.9, 7.1 Hz, 2H, –CH2–), 3.99 (q, J = 6.1 Hz, 2H, –CH2–), 2.98 (ddt, J = 13.2, 11.1, 6.1 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 156.5 (d, J = 249.8 Hz), 148.7, 143.0 (q, J = 3.8 Hz), 137.6 (q, J = 3.5 Hz), 132.1, 131.2, 130.6 (d, J = 2.8 Hz), 129.4 (q, J = 34.1 Hz), 125.0 (d, J = 4.8 Hz), 122.1 (q, J = 273.5 Hz), 121.8 (d, J = 18.0 Hz), 118.7 (d, J = 15.2 Hz), 51.3, 50.2, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.58, −117.98. HRMS: calculated for C16H13O2N2Cl2F4S [M + H]+: 443.00054; found: 442.99860.
3-Chloro-N-(2-((4-isopropylbenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G3). Yield 45%; faint yellow solid; mp 180–181 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.44 (t, J = 5.4 Hz, 1H, –CO–NH–), 8.06 (d, J = 1.4 Hz, 1H, pyridine-H), 7.26–7.19 (m, 4H, Ar–H), 4.05 (q, J = 13.0 Hz, 2H, –CH2–), 4.00–3.92 (m, 2H, –CH2–), 3.02 (ddd, J = 13.1, 7.4, 5.5 Hz, 1H, –CH–), 2.93–2.78 (m, 2H, –CH2–), 1.24 (d, J = 6.9 Hz, 6H, –CH3). 13C NMR (100 MHz, CDCl3) δ 162.7, 149.5, 149.0, 143.1 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz), 132.0, 130.1, 129.3 (q, J = 34.0 Hz), 127.2, 126.4, 122.1 (q, J = 273.5 Hz), 58.2, 49.2, 34.5, 33.9, 23.9. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C19H21O2N2ClF3S [M + H]+: 433.09589; found: 433. 09573.
N-(2-((2-Bromo-4-fluorobenzyl)sulfinyl)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (G4). Yield 38%; faint yellow solid; mp 184–185 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.38 (t, J = 5.1 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.4 Hz, 1H, pyridine-H), 7.39 (ddd, J = 8.1, 7.5, 4.2 Hz, 2H, Ar–H), 7.11–6.99 (m, 1H, Ar–H), 4.22 (dd, J = 46.5, 13.1 Hz, 2H, –CH2–), 4.01 (ddd, J = 8.0, 5.5, 1.4 Hz, 2H, –CH2–), 3.25–2.84 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 162.4 (d, J = 253.3 Hz), 148.8, 143.1 (q, J = 3.9 Hz), 137.62 (q, J = 3.6 Hz), 133.5 (d, J = 8.6 Hz), 132.1, 129.4 (q, J = 34.1 Hz), 125.8 (d, J = 3.7 Hz), 125.2 (d, J = 9.7 Hz), 122.1 (q, J = 273.4 Hz), 120.7 (d, J = 24.7 Hz), 115.3 (d, J = 21.2 Hz), 57.7, 49.9, 34.5. 19F NMR (376 MHz, CDCl3) δ −62.58, −110.16. HRMS: calculated for C16H13O2N2BrClF4S [M + H]+: 489.95003; found: 489.95001.
N-(2-((3-Bromobenzyl)sulfinyl)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (G5). Yield 50%; white solid; mp 158–159 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.0 Hz, 1H, pyridine-H), 8.41 (t, J = 5.4 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.3 Hz, 1H, pyridine-H), 7.52–7.45 (m, 2H, Ar–H), 7.33–7.19 (m, 2H, Ar–H), 4.06–3.99 (m, 2H, –CH2–), 3.99–3.93 (m, 2H, –CH2–), 3.21–2.69 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 148.7, 143.1 (q, J = 3.8 Hz), 137.7 (q, J = 3.5 Hz), 133.0, 132.1, 131.8, 131.7, 130.6, 129.4 (q, J = 34.1 Hz), 128.8, 123.0, 122.1 (q, J = 273.4 Hz), 57.7, 49.8, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C16H14O2N2BrClF3S [M + H]+: 468.95945; found: 468.95941.
3-Chloro-N-(2-((2,6-difluorobenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G6). Yield 40%; white solid; mp 144–145 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.43 (t, J = 5.3 Hz, 1H, –CO–NH–), 8.06 (d, J = 1.3 Hz, 1H, pyridine-H), 7.33 (tt, J = 8.4, 6.5 Hz, 1H, Ar–H), 7.04–6.91 (m, 2H, Ar–H), 4.30–4.15 (m, 2H, –CH2–), 4.01 (ddd, J = 8.4, 5.3, 1.6 Hz, 2H, –CH2–), 3.20–2.86 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 161.5 (d, J = 250.7 Hz), 161.5 (d, J = 250.7 Hz), 148.9, 143.1 (q, J = 3.9 Hz), 137.5 (q, J = 3.6 Hz), 132.0, 130.8 (t, J = 10.2 Hz), 129.3 (q, J = 34.0 Hz), 122.1 (q, J = 273.5 Hz), 111.8 (q, J = 25.3 Hz), 106.3 (t, J = 19.4 Hz), 50.2, 45.8, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.57, −112.09. HRMS: calculated for C16H13O2N2ClF5S [M + H]+: 427.03009; found: 427.03006.
3-Chloro-N-(2-((2-methylbenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G7). Yield 52%; white solid; mp 136–137 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 0.9 Hz, 1H, pyridine-H), 8.45 (t, J = 5.1 Hz, 1H, –CO–NH–), 8.06 (d, J = 1.3 Hz, 1H, pyridine-H), 7.26–7.17 (m, 4H, Ar–H), 4.15 (dd, J = 52.1, 12.9 Hz, 2H, –CH2–), 4.05–3.91 (m, 2H, –CH2–), 3.13–2.87 (m, 2H, –CH2–), 2.41 (s, 3H, –CH3). 13C NMR (100 MHz, CDCl3) δ 162.7, 149.0, 143.1 (q, J = 3.8 Hz), 137.5 (q, J = 7.2 Hz), 137.4, 132.0, 131.1, 131.0, 129.3 (q, J = 34.0 Hz), 128.9, 128.0, 126.7, 122.1 (q, J = 273.5 Hz), 57.2, 49.6, 34.5, 19.9. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C17H17O2N2ClF3S [M + H]+: 405.06459; found: 495.06454.
3-Chloro-5-(trifluoromethyl)-N-(2-((2-(trifluoromethyl)benzyl)sulfinyl)ethyl)picolinamide (G8). Yield 35%; white solid; mp 131–132 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.0 Hz, 1H, pyridine-H), 8.43 (t, J = 5.2 Hz, 1H, –CO–NH–), 8.07 (d, J = 1.3 Hz, 1H, pyridine-H), 7.72 (d, J = 7.8 Hz, 1H, Ar–H), 7.61–7.54 (m, 2H, Ar–H), 7.49 (td, J = 8.2, 4.7 Hz, 1H, Ar–H), 4.29–4.12 (m, 2H, –CH2–), 4.10–3.94 (m, 2H, –CH2–), 3.21–2.93 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.7, 148.8, 143.1 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz), 132.9, 132.3, 132.1, 129.3 (q, J = 34.1 Hz), 129.1 (q, J = 30.1 Hz), 128.8, 128.8, 126.7 (q, J = 5.5 Hz), 124.1 (q, J = 273.9 Hz), 122.1 (q, J = 273.5 Hz), 56.2, 50.7, 34.5. 19F NMR (376 MHz, CDCl3) δ −58.50, −62.58. HRMS: calculated for C17H14O2N2ClF6S [M + H]+: 459.03632; found: 459.03586.
3-Chloro-N-(2-((2-chlorobenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G9). Yield 45%; faint yellow solid; mp 127–128 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.42 (t, J = 5.2 Hz, 1H, –CO–NH–), 8.06 (d, J = 1.3 Hz, 1H, pyridine-H), 7.46–7.38 (m, 2H, Ar–H), 7.34–7.28 (m, 2H, Ar–H), 4.26 (dd, J = 29.2, 13.0 Hz, 2H, –CH2–), 4.08–3.92 (m, 2H, –CH2–), 3.20–2.85 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.7, 148.9, 143.1 (q, J = 3.9 Hz), 137.6 (q, J = 3.6 Hz), 134.6, 132.5, 132.1, 130.1, 130.0, 129.3 (q, J = 34.0 Hz), 127.8, 127.4, 122.1 (q, J = 273.5 Hz), 56.1, 49.8, 34.5. 19F NMR (376 MHz, CDCl3) δ −62.55. HRMS: calculated for C15H14O3N2Cl2F3S [M + H]+: 441.00488; found: 441.00427.
N-(2-((4-(tert-Butyl)benzyl)sulfinyl)ethyl)-3-chloro-5-(trifluoromethyl)picolinamide (G10). Yield 55%; faint creamy yellow solid; mp 131–133 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 0.9 Hz, 1H, pyridine-H), 8.45 (t, J = 5.4 Hz, 1H, –CO–NH–), 8.06 (d, J = 1.2 Hz, 1H, pyridine-H), 7.40 (d, J = 8.2 Hz, 2H, Ar–H), 7.23 (d, J = 8.2 Hz, 2H, Ar–H), 4.05 (q, J = 13.0 Hz, 2H, –CH2–), 4.00–3.93 (m, 2H, –CH2–), 3.09–2.78 (m, 2H, –CH2–), 1.31 (s, 9H, –C(CH3)3). 13C NMR (100 MHz, CDCl3) δ 162.8, 151.7, 149.0, 143.1 (q, J = 3.8 Hz), 137.6 (q, J = 3.6 Hz), 132.0, 129.8, 129.3 (d, J = 34.0 Hz), 126.1, 126.1, 122.1 (q, J = 273.4 Hz), 58.1, 49.2, 34.7, 34.5, 31.3. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C20H23O2N2ClF3S [M + H]+: 447.1154; found: 477.11038.
3-Chloro-N-(2-(((6-chloropyridin-3-yl)methyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G11). Yield 72%; soil yellow solid; mp 136–137 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.38 (t, J = 5.2 Hz, 1H, –CO–NH–), 8.35 (d, J = 2.3 Hz, 1H, pyridine-H), 8.08 (d, J = 1.3 Hz, 1H, pyridine-H), 7.68 (dd, J = 8.2, 2.4 Hz, 1H, pyridine-H), 7.37 (d, J = 8.2 Hz, 1H, pyridine-H), 4.16–3.86 (m, 4H, –CH2–), 3.25–2.76 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.9, 151.9, 150.7, 148.4, 143.0 (q, J = 3.8 Hz), 140.4, 137.8 (q, J = 3.6 Hz), 132.2, 129.5 (q, J = 34.0 Hz), 124.7, 124.5, 122.1 (q, J = 273.5 Hz), 53.9, 50.3, 34.3. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C15H13O2N3Cl2F3S [M + H]+: 426.00521; found: 426.00473.
3-Chloro-N-(2-((4-cyanobenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G12). Yield 39%; pale yellow solid; mp 172–173 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H, pyridine-H), 8.36 (t, J = 5.3 Hz, 1H, –CO–NH–), 8.08 (s, 1H, pyridine-H), 7.69 (d, J = 7.7 Hz, 2H, Ar–H), 7.45 (d, J = 7.9 Hz, 2H, Ar–H), 4.09 (dd, J = 53.4, 13.0 Hz, 2H, –CH2–), 4.00–3.83 (m, 2H, –CH2–), 3.31–2.77 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.9, 148.5, 143.0 (q, J = 3.8 Hz), 137.8 (q, J = 3.6 Hz), 135.0, 132.7, 132.2, 131.0, 129.5 (q, J = 34.1 Hz), 122.1 (q, J = 273.5 Hz), 118.3, 112.6, 57.6, 50.3, 34.3. 19F NMR (376 MHz, CDCl3) δ −62.56. HRMS: calculated for C17H11O2N3Cl2F3S [M + H]+: 432.03910; found: 432.03787.
3-Chloro-N-(2-((3,5-difluorobenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G13). Yield 34%; soil white solid; mp 154–155 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 1.1 Hz, 1H, pyridine-H), 8.40 (t, J = 5.5 Hz, 1H, –CO–NH–), 8.08 (d, J = 1.4 Hz, 1H, pyridine-H), 6.93–6.85 (m, 2H, Ar–H), 6.82 (ddt, J = 11.2, 8.9, 2.5 Hz, 1H, Ar–H), 4.11–3.87 (m, 4H, –CH2–), 3.23–2.80 (m, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 163.2 (d, J = 250.3 Hz), 163.0 (d, J = 250.3 Hz), 162.9, 148.6, 143.1 (q, J = 3.9 Hz), 137.7 (q, J = 3.5 Hz), 133.2 (t, J = 9.6 Hz), 132.2, 129.5 (q, J = 34.1 Hz), 122.1 (q, J = 273.4 Hz), 113.2 (dd, J = 11.4 Hz, 25.9 Hz), 104.3 (t, J = 25.1 Hz), 57.5, 50.2, 34.3. 19F NMR (376 MHz, CDCl3) δ −62.58, −108.29. HRMS: calculated for C16H13O2N2ClF5S [M + H]+: 427.03009; found: 427.02844.
3-Chloro-N-(2-(((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G14). Yield 41%; white solid; mp 126–127 °C. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 1.0 Hz, 1H, pyridine-H), 8.62 (t, J = 5.1 Hz, 1H, –CO–NH–), 8.35 (d, J = 5.6 Hz, 1H, pyridine-H), 8.06 (d, J = 1.4 Hz, 1H, pyridine-H), 6.70 (d, J = 5.6 Hz, 1H, pyridine-H), 4.51–4.25 (m, 4H, –CH2–), 4.02 (dd, J = 11.8, 5.9 Hz, 2H, –CH2–), 3.50–2.79 (m, 2H, –CH2–), 2.32 (s, 3H, –CH3). 13C NMR (100 MHz, CDCl3) δ 162.7, 161.9, 150.9, 149.2, 148.4, 143.0 (q, J = 3.8 Hz), 137.5 (q, J = 3.5 Hz), 132.0, 129.2 (q, J = 34.0 Hz), 122.8 (q, J = 277.7 Hz), 122.141 (q, J = 273.5 Hz), 123.4, 105.9, 65.4 (q, J = 36.5 Hz), 57.5, 50.2, 34.3, 11.2. 19F NMR (376 MHz, CDCl3) δ −62.57, −73.78. HRMS: calculated for C18H17O3N3ClF6S [M + H]+: 504.05779; found: 504.05603.
3-Chloro-N-(2-((3,4,4-trifluorobut-3-en-1-yl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G15). Yield 30%; pale yellow solid; mp 100–101 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (s, 1H, pyridine-H), 8.45 (s, 1H, –CO–NH–), 8.08 (d, J = 1.2 Hz, 1H, pyridine-H), 4.01 (dd, J = 11.8, 5.9 Hz, 2H, –CH2–), 3.31–2.94 (m, 2H, –CH2–), 2.86 (ddd, J = 12.6, 10.7, 8.4 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3) δ 162.8, 153.1 (ddd, J = 288.2, 275.3, 45.7 Hz), 148.6, 143.1 (q, J = 3.8 Hz), 137.7 (q, J = 3.5 Hz), 132.2, 129.5 (q, J = 34.4 Hz), 126.3 (ddd, J = 234.8, 53.5, 17.5 Hz), 122.1 (q, J = 273.5 Hz), 51.2, 48.1, 34.5, 19.8 (dd, J = 22.2, 2.3 Hz). 19F NMR (376 MHz, CDCl3) δ −62.59, −102.12 (dd, J = 82.1, 33.0 Hz), −121.38 (ddd, J = 115.7, 82.5, 4.4 Hz), −175.45 (dd, J = 114.8, 33.0 Hz). HRMS: calculated for C13H11O2N3Cl2F3S2 [M + H]+: 431.96163; found: 431.96161.
3-Chloro-N-(2-((2,5-difluorobenzyl)sulfinyl)ethyl)-5-(trifluoromethyl)picolinamide (G16). Yield 30%; white solid; mp 123–125 °C. 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 0.9 Hz, 1H), 8.40 (t, J = 4.8 Hz, 1H), 8.07 (d, J = 1.4 Hz, 1H), 7.18–6.93 (m, 2H), 4.08 (dd, J = 36.3, 13.2 Hz, 2H), 3.98 (dd, J = 12.0, 6.1 Hz, 2H), 3.17–2.82 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 162.8, 158.5 (dd, J = 244.3, 2.3 Hz), 157.0 (dd, J = 243.6, 2.6 Hz), 148.7, 143.0 (q, J = 3.8 Hz), 137.6 (q, J = 3.5 Hz), 132.1, 129.4 (q, J = 34.1 Hz), 122.1 (q, J = 273.4 Hz), 118.9 (d, J = 3.6 Hz), 118.6 (d, J = 3.6 Hz), 118.4 (dd, J = 17.9, 8.3 Hz), 117.0 (td, J = 24.4, 8.6 Hz), 50.8, 50.0, 34.4. 19F NMR (376 MHz, CDCl3) δ −62.57, −117.43 (d, J = 17.9 Hz), −122.28 (d, J = 17.8 Hz). HRMS: calculated for C16H13O2N2ClF5S [M + H]+: 427.03009; found: 427.02939.
| Activity: I = (C − T)/C × 100%, |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21762012), and Program of Introducing Talents to Chinese Universities (111 Program, D20023), and the S&T Planning Project of Guizhou Province (No. [2017]1402, [2017]5788).Notes and references
- F. P. Carvalho, Food Energy Secur., 2017, 6, 48 CrossRef.
- R. N. Strange and P. R. Scott, Annu. Rev. Phytopathol., 2005, 43, 83 CrossRef CAS.
- S. H. Ou, Rice Diseases, Commonwealth Agricultural Bureau, 1985 Search PubMed.
- N. Huang, E. R. Angeles, J. Domingo, G. Magpantay, S. Singh, G. Zhang, N. Kumaravadivel, J. Bennett and G. S. Khush, Theor. Appl. Genet., 1997, 95, 313 CrossRef CAS.
- A. C. Hayward, Annu. Rev. Phytopathol., 1991, 29, 65 CrossRef CAS.
- G. Kemmitt, T. C. Sparks, J. E. Hunter, B. A. Lorsbach, G. Hanger, R. E. Gast and R. J. Bryant, J. Agric. Food. Chem., 2018, 66, 10337 CrossRef.
- T. H. Kim, J. W. Jeong, J. H. Song, K. R. Lee, S. Ahn, S. H. Ahn, S. S. Kim and T. S. Koo, Arch. Pharmacal Res., 2015, 38, 2076 CrossRef CAS.
- M. I. Ansari, M. M. Khan, M. Saquib, S. Khatoon and M. K. Hussain, Future Med. Chem., 2018, 10, 1241 CrossRef CAS.
- J. Zhao and X. Jiang, Chin. Chem. Lett., 2018, 29, 1079 CrossRef CAS.
- Q. Jing, X. Hu, M. Yanzi, J. H. Mu, W. W. Liu, F. X. Xu, Z. L. Li, J. Bai, H. M. Hua and D. H. Li, Mar. Drugs, 2019, 17, 384 CrossRef CAS.
- S. Pathania, R. K. Narang and R. K. Rawal, Eur. J. Med. Chem., 2019, 180, 486 CrossRef CAS.
- R. R. Singhaus, R. C. Bernotas, R. Steffan, E. Matelan, E. Quinet, P. Nambi, I. Feingold, C. Huselton, A. Wilhelmsson, A. Goos-Nilsson and J. Wrobel, Bioorg. Med. Chem. Lett., 2010, 20, 521 CrossRef CAS.
- B. Prasad, R. Adepu, S. Sandra, D. Rambabu, G. Krishna, R. Rama, C. Malla, G. S. Deora, P. Misra and M. Pal, Chem. Commun., 2012, 48, 10434 RSC.
- A. Kallinen, R. Boyd, S. Lane, R. Bhalla, K. Mardon, D. H. R. Stimson, E. L. Werry, R. Fulton, M. Connor and M. Kassiou, Org. Biomol. Chem., 2019, 17, 5086 RSC.
- F. Z. Karadayi, M. Yaman, M. M. Kisla, A. G. Keskus, O. Konu and Z. A. Alagoz, Bioorg. Chem., 2020, 100, 103929 CrossRef CAS.
- S. Mondal, K. Mahato, N. Arora, D. Kankane, U. P. Singh, S. Ali, A. H. Khan, S. S. Ghosh and A. T. Khan, Org. Biomol. Chem., 2020, 18, 4104 RSC.
- T. Wang, T. Peng, X. X. Wen, G. Wang, Y. B. Sun, S. C. Liu, S. G. Zhang and L. Wang, Molecules, 2019, 24, 4034 CrossRef CAS.
- G. L. Regina, M. C. Edler, A. Brancale, S. Kandil, A. Coluccia, F. Piscitelli, E. Hamel, G. D. Martino, R. Matesanz, J. F. Diaz, A. I. Scovassi, E. Prosperi, A. Lavecchia, E. Novellino, M. Artico and R. Silvestri, J. Med. Chem., 2007, 50, 2865 CrossRef.
- R. Ragno, A. Coluccia, G. L. Regina, G. D. Martino, F. Piscitelli, A. Lavecchia, E. Novellino, A. Bergamini, C. Ciaprini, A. Sinistro, G. Maga, E. Crespan, M. Artico and R. Silvestri, J. Med. Chem., 2006, 49, 3172 CrossRef CAS.
- V. Famiglini, G. L. Regina, A. Coluccia, S. Pelliccia, A. Brancale, G. Maga, E. Crespan, R. Badia, E. Riveira-Munoz, J. A. Este, R. Ferretti, R. Cirilli, C. Zamperini, M. Botta, D. Schols, V. Limongelli, B. Agostino, E. Novellino and R. Silvestri, J. Med. Chem., 2014, 57, 9945 CrossRef CAS.
- D. Alia and K. F. Frank, Expert Opin. Drug Saf., 2009, 8, 119 CrossRef.
- B. Shu, Q. Yu, D. X. Hu, T. Che, S. S. Zhang and D. Li, Bioorg. Med. Chem. Lett., 2020, 30, 126925 CrossRef CAS.
- K. L. Manasa, S. Pujitha, A. Sethi, M. Arifuddin, M. Alvala, A. Angeli and C. T. Supuran, Metabolites, 2020, 10, 136 CrossRef CAS.
- G. T. Gibney and J. S. Zager, J. Drug Metab. Toxicol., 2013, 9, 893 CrossRef CAS.
- P. Koelblinger, O. Thuerigen and R. Dummer, Curr. Opin. Oncol., 2018, 30, 125 CrossRef CAS.
- W. F. Richard, B. Christine, C. C. Chi, C. Stella, D. Denis, D. Daniel, E. Diane, Y. G. Jacques, G. Yves, G. Robert, M. G. Gillian, R. Denis, S. Chantal, Z. Y. Wang, E. Wong, D. Visco, L. J. Xu and R. N. Young, Bioorg. Med. Chem. Lett., 1998, 8, 2777 CrossRef.
- P. Srinivas, P. Manojit and Y. K. Rao, Tetrahedron, 2003, 59, 7915 CrossRef.
- W. M. Xu, S. Z. Li, M. He, S. Yang, X. Y. Li and P. Li, Bioorg. Med. Chem. Lett., 2013, 23, 5821 CrossRef CAS.
- P. Y. Wang, L. Zhou, J. Zhou, Z. B. Wu, W. Xue, B. A. Song and S. Yang, Bioorg. Med. Chem. Lett., 2016, 26, 1214 CrossRef CAS.
- Y. T. Zheng, T. T. Zhang, P. Y. Wang, Z. B. Wu, L. Zhou, I. Q. Ye, X. Zhou, M. He and S. Yang, Chin. Chem. Lett., 2017, 28, 253 CrossRef CAS.
- P. Li, J. L. Zhou, Y. Liu and X. Wang, Chem. Pap., 2020 DOI:10.1007/s11696-020-01271-6.
- I. Grib, M. Berredjem, K. O. Rachedi, S. E. Djouad, S. Bouacida, R. Bahadi, T. S. Ouk, M. Kadri, T. B. Hadda and B. Belhani, J. Mol. Struct., 2020, 1217, 128423 CrossRef CAS.
- M. Z. Zhang, N. Mulholland, D. Beattie, D. Irwin, Y. C. Gu, G. F. Yang and C. John, Eur. J. Med. Chem., 2013, 63, 22 CrossRef CAS.
- S. Y. Ke, W. Fang, W. B. Huang, Z. G. Zhang, L. Q. Shi, Z. Y. Wan, K. M. Wang, C. X. Cao and D. Y. Huang, Bioorg. Med. Chem. Lett., 2020, 30, 127245 CrossRef CAS.
- M. A. Gonzalez, D. B. Gorman, C. T. Hamilton and G. A. Roth, Org. Process Res. Dev., 2008, 12, 301 CrossRef CAS.
- J. M. Babcock, C. B. Gerwick, J. X. Huang, M. R. Loso, G. Nakamura, S. P. Nolting, R. B. Rogers, T. C. Sparks, J. Thomas, G. B. Watson and Y. Zhu, Pest Manage. Sci., 2011, 67, 328 CrossRef CAS.
- Z. B. Yang, P. Li, Y. J. He, J. Luo, J. Zhou, Y. H. Wu and L. T. Chen, Chem. Pap., 2020, 74, 1621 CrossRef CAS.
- S. Patai, Z. Rappoport and C. Stirling, The Chemistry of Sulphones and Sulphoxides, John Wiley & Sons, Ltd, 2006 Search PubMed.
- A.-N. R. Alba, X. Companyó and R. Rios, Chem. Soc. Rev., 2010, 39, 2018 RSC.
- M. Nielsen, C. B. Jacobsen, N. Holub, M. W. Paixão and K. A. Jørgensen, Angew. Chem., Int. Ed., 2010, 49, 2668 CrossRef CAS.
- T. Haga, Y. Tsujii, K. Hayashi, F. Kimura, N. Sakashita and K. Fujikawa, ACS Symp. Ser., 1991, 443, 107 CrossRef CAS.
- I. Katsuyama, Yuki Gosei Kagaku Kyokaishi, 2009, 67, 992 CrossRef CAS.
- B. Adam, J. F. Edmunds, D. E. Daniel, H. G. Roger, J. Olivier and S. Juergen, Pest Manage. Sci., 2018, 74, 1228 CrossRef.
- F. Z. Xu, Y. Y. Wang, D. X. Luo, G. Yu, S. X. Guo, H. Fu, Y. H. Zhao and J. Wu, RSC Adv., 2018, 8, 6306 RSC.
- F. Z. Xu, Y. Y. Wang, D. X. Luo, G. Yu, Y. k. Wu, A. L. Dai, Y. H. Zhao and J. Wu, ChemistrySelect, 2018, 3, 2795 CrossRef CAS.
- A. L. Dai, S. X. Guo, C. H. Li, R. F. Zhang and J. Wu, Mod. Agrochem., 2020, 19, 21 Search PubMed.
- P. Li, D. Y. Hu, D. D. Xie, J. X. Chen, L. H. Jin and B. A. Song, J. Agric. Food Chem., 2018, 66, 3093 CrossRef CAS.
- P. Li, P. Y. Tian, Y. Z. Chen, X. P. Song, W. Xue, L. H. Jin, D. Y. Hu and B. A. Song, Pest Manage. Sci., 2018, 74, 844 CrossRef CAS.
- P. Dalgaard, T. Ross, L. Kamperman, K. Neumeyer and T. A. McMeekin, Int. J. Food Microbiol., 1994, 23, 391 CrossRef CAS.
- W. S. Abbott, J. Am. Mosq. Control Assoc., 1987, 3, 302 CAS.
- Q. Q. Zhao, Y. Q. Li, L. X. Xiong and Q. M. Wang, J. Agric. Food Chem., 2010, 58, 4992 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The copies of 1H NMR, 19F NMR, 13C NMR and HR-MS spectrograms for all the synthesized compounds. See DOI: 10.1039/d0ra07301f |
| ‡ Co-first author for the manuscript. |
| This journal is © The Royal Society of Chemistry 2020 |