Open Access Article
Open Access ArticleA rational design of a cancer-specific and lysosome-targeted fluorescence nanoprobe for glutathione imaging in living cells†
Hong
Wang
 ab,
Peisheng
Zhang
ab,
Peisheng
Zhang
 *ac,
Chonghua
Zhang
a,
Shu
Chen
a,
Rongjin
Zeng
a,
Jiaxi
Cui
*ac,
Chonghua
Zhang
a,
Shu
Chen
a,
Rongjin
Zeng
a,
Jiaxi
Cui
 bd and
Jian
Chen
bd and
Jian
Chen
 *ae
*ae
aKey Laboratory of Theoretical Organic Chemistry and Function Molecule, Ministry of Education, Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers, Hunan Province College Key Laboratory of QSAR/QSPR, Hunan Provincial Key Lab of Advanced Materials for New Energy Storage and Conversion, School of Chemistry and Chemical Hunan University of Science and Technology, Xiangtan 411201, P. R. China. E-mail: pshzhang07@gmail.com; cj0066@gmail.com
bInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, Sichuan 610054, China
cState Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan University, Changsha 410082, China
dINM-Leibniz Institute for New Materials, Campus D2 2, 66123 Saarbrícken, Germany
eState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, P. R. China
First published on 18th July 2020
Abstract
Developing a versatile probe for targeting the lysosomes of specific cancer cells and subsequently detecting glutathione (GSH) levels is critical in disclosing the roles of GSH in the lysosomal oxidative stress of cancer cells. Herein, we demonstrate an efficient strategy for the preparation of a dual-targeting (both cancer cell- and lysosome-targeting) fluorescence nanoprobe (DTFN) that enables the imaging of GSH in the lysosomes of specific cancer cells. The nanoprobe (DTFN) is obtained by combining folic acid (FA)-modified photostable aggregation-induced emission dots with GSH-responsive manganese dioxide (MnO2) nanosheets via electrostatic interactions. DTFN has outstanding characteristics of good water dispersity, delightful photostability, shorter responsive time (∼5 min) and wide pH-response range. Intracellular experiments showed that the as-prepared DTFN could be preferentially internalized into a folate receptor (FR)-positive cancer cells via the FR-mediated endocytosis. Subsequently, with the aid of the positively charged amino moiety of the nanoprobe, DTFN can selectively accumulate in lysosomes and successfully achieve the real-time imaging of the lysosomal GSH levels in FR-positive cancer cells. This study highlights a strategy to design a versatile dual-targeting fluorescence probe for enhanced cancer imaging.
Introduction
Cancer, as a destructive and prevalent disease worldwide, seriously affects human health and arouses widespread concern. As important acidic digestive organelles of the endocytic and autophagic pathways in cancer cells, lysosomes are a crucial target of uncontrolled oxidative processes to free radical damage.1,2 For lysosomal oxidative stress, glutathione (GSH) as an effective cellular antioxidant plays important roles in the homeostasis of oxidative stress state because it can effectively eliminate reactive oxygen species.3,4 Thus, there is an indigenous incentive to design and prepare practical tools for efficiently detecting lysosomal GSH levels within cancer cells to reveal the roles of GSH in the lysosomal oxidative stress of cancer cells.On account of numerous advantages including non-invasive, simple preparation, high sensitivity, and high spatial resolution, fluorescence probes coupled with fluorescence microscopy imaging have become a powerful supporting tool for the detection of various analytes.5–25 So far, much effort has gone into the development of unique GSH fluorescence probes.26–28 However, most of them were either cancer cells- or subcellular organelle-specific targeting GSH fluorescence probes;29–38 however, single targeting systems were still unable to achieve efficient GSH detection. It is known that to realize the goal of efficiently detecting GSH in lysosome of specific cancer cells, an ideal GSH fluorescence probe should be constructed by combining specific cancer cell- and lysosome-targeting functions together with a GSH probe.
For the dual-targeting (both cancer cell- and lysosome-targeting) GSH fluorescence probe system, the one key challenge is to integrate the targeting ligand, fluorophore, GSH-responsive group and other functional groups into one system. In general, because of the limited number of attachment sites, it is difficult to incorporate effectively multiple functional groups in one small organic molecule-based fluorophore. Alternatively, polymeric nanoparticles formed by amphiphilic diblock copolymers are promising candidates for constructing a dual-targeting GSH fluorescence probe due to the flexible structural design, suitable water solubility, favorable biocompatibility and so on.39–46 Up to now, several polymeric nanoparticle-based dual-targeting nanoprobes have been developed for cancer imaging and therapy.47–54 However, to the best of our knowledge, dual-targeting fluorescence polymeric nanoprobes for efficiently detecting lysosomal GSH within specific cancer cells have not yet been reported.
Hence, a simple and versatile dual-targeting fluorescence nanoprobe (DTFN) is developed for efficiently detecting lysosomal GSH in specific cancer cells, as depicted in Scheme 1. As for this system, the folic acid (FA)-modified photostable aggregation-induced emission (AIE) dots were first synthesized via aco-precipitation strategy.
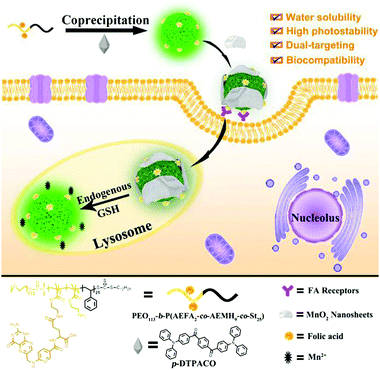 | ||
| Scheme 1 Schematic illustration of dual-targeting fluorescence nanoprobe (DTFN) for efficiently detecting lysosomal GSH within cancer cells. | ||
As the nanocarrier, the amphiphilic block copolymers show multiple purposes: (1) the incorporation of polyethylene glycol chains could not only possess good water dispersibility and excellent biocompatibility but also enhance the photostability of AIE fluorogen for a long time; (2) the efficient introduction of a cancer targeting unit (folic acid, FA) into nanoprobe to enhance the effective uptake of the folate receptor (FR) over-expressed cancer cells and efficiently reduce the nonspecific uptake by normal cells;47,55–57 (3) the positively charged amino moiety enables the nanoparticles enter into lysosomes.45,58 Subsequently, GSH-responsive manganese dioxide (MnO2) nanosheets, which own the ability to quench the fluorescence of the AIE fluorogen, are absorbed onto the surface of AIE dots based on electrostatic interaction to obtain DTFN. Our results suggest that the dual-targeting strategy could allow DTFN to selectively internalize into the lysosomes of FR-positive cancer cells and successfully achieve efficient GSH detection.
Experimental section
Materials and apparatus
All the solvents and chemical materials mentioned in this study were purchased and used directly. 1,4-Phenylenebis((4-(diphenylamino)phen-yl)methanone) (p-DTPACO) and the PEO macro-RAFT agent (PEO113-TTC) were prepared via a procedure reported in our previous study.45,59 MnO2 nanosheets were synthesized according to the reported methods.60Results and discussion
Scheme 1 illustrated the design strategy of DTFN for the efficient lysosomal GSH detection within specific cancer cells. First, the amphiphilic block copolymer PEO113-b-P(AEFA2-co-AEMH9-co-St25) containing both folic acid (FA) and amino group was synthesized through the reversible addition fragmentation chain transfer (RAFT) polymerization and the further chemical graft reactions (Scheme S1, ESI†). The related intermediates were confirmed via1H NMR, GPC, and absorption spectra (Fig. S1–S4 and Table S1, ESI†).Subsequently, the FA-modified AIE dots with positive charge (+11.2 mV) were synthesized using PEO113-b-P(AEFA2-co-AEMH9-co-St25) and p-DTPACO via a co-precipitation strategy.45,59 The data based on the transmission electron microscopy (TEM) and dynamic light scattering (DLS) showed that the morphology of FA-modified AIE dots was spherical and the average size was about 40 nm (Fig. S5A and S6, ESI†). Finally, the as-prepared MnO2 nanosheets with negative charge were loaded on the surface of the positively charged FA-modified AIE dots via electrostatic interaction to obtain DTFN (Fig. S7, ESI†).60 The TEM data also confirmed the successful synthesis of DTFN (Fig. S5B, ESI†). For the FA-modified AIE dots and DTFN, the obvious band at 1630 cm−1 of the amine group (–NH2) can be seen in Fig. S8 (ESI†), indicating that they contained the positively charged amino moiety (–NH2). In addition, compared with the FA-modified AIE dots, the band (Mn–O) at 610 cm−1 of DTFN obviously varied (Fig. S8, ESI†). The above results clearly demonstrated that the MnO2 nanosheets were loaded on the surface of the FA-modified AIE dots to obtain DTFN.
Interestingly, the fluorescence emission of the FA-modified AIE dots overlapped with the absorbance spectra of the MnO2 nanosheets (Fig. S9, ESI†), leading to the occurrence of the fluorescence resonance energy transfer (FRET) effect from AIE dots to the MnO2 nanosheets.60,61 Thus, an obvious quenched fluorescence can be visualized upon the addition of MnO2 nanosheets to the solution of FA-modified AIE dots. In addition, a maximum fluorescence quenching degree of FA-modified AIE dots up to 78% can be observed upon the addition of 300 μg mL−1 MnO2 nanosheets (Fig. S10, ESI†).
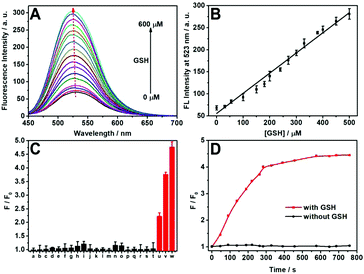
First, the fluorescence titration of DTFN to GSH was performed in a PBS buffered solution (pH 7.4). The nanoprobe DTFN itself displayed a weak fluorescence owing to the occurrence of the FRET effect from AIE dots to MnO2 nanosheets. However, the MnO2 nanosheets reduced to Mn2+ with the addition of GSH, and thus inhibit the FRET process, resulting in the recovery of green fluorescence (Fig. 1A). In addition, a class linear relationship (R2 = 0.9855) can be observed by plotting I523 and GSH concentration (0–500 μM) (Fig. 1B) and detection limit (LOD) was achieved down to 1.03 μM (S/N = 3) (Fig. S11, ESI†). Moreover, the photos of DTFN towards different GSH concentrations were observed using visible light and UV light radiation. As depicted in Fig. S12 (ESI†), with the increase in the GSH concentration (0–600 μM), the fluorescence of DTFN revealed an obvious increase, accompanying with the obvious color change of DTFN from yellow to colorless.
Subsequently, the selectivity of DTFN for GSH over various other analytes was carried out. As depicted in Fig. 1C, no significant change in the emission ratio (F/F0) can be visualized when DTFN was treated with other analytes including ions (Na+, K+, Co2+, Ca2+, Mg2+, Ni2+, Cu2+, and Fe3+), amino acids (Ser, Asn, Thr, Gln, Gly, Ala, Arg, His, and Met), BSA and glucose. However, GSH can trigger a large enhancement in the emission ratio (F/F0). Notably, various literatures indicated that the content of GSH in cancer cells was about 1000-fold more than that of cysteine (Cys) or homocysteine (Hcy).62–64 In view of these reports, a contrast experiment on the fluorescence intensity ratio (F/F0) was conducted between GSH, Cys and Hcy. As exhibited in Fig. S13 (ESI†), both Cys and Hcy revealed tiny effects on the detection of GSH even their concentration (60 μM) was as high as one tenth relative to GSH (600 μM). These results demonstrated the good selectivity of DTFN towards GSH against other various analytes. In addition, the response time of DTFN towards GSH was less than 5 min, which is benefitial for the imaging of endogenous GSH in live cells.
Next, the pH-dependent fluorescent intensity (I523) of DTFN with and without GSH was also explored (Fig. S14, ESI†). Without GSH, a relatively weakened fluorescence intensity (I523) can be observed at the pH range from 4.0 to 9.0. However, a significant fluorescence enhancement of DTFN with GSH was observed in a wide pH range from 4.0 to 9.0, indicating that DTFN was an excellent tool for sensing GSH in the physiological environment. The photostability of DTFN without and with GSH was also conducted under the irradiation of UV light (365 nm, 2.8 mW cm−2, Fig. S15, ESI†). The results revealed that the emission intensity (I523) of DTFN with GSH revealed only a slight change (<5%) upon UV irradiation for 90 min, indicating that DTFN had excellent photostability. In addition, to investigate the practicability of DTFN, the adscititious GSH contents in the blood serum sample were detected by the DTFN probe. As exhibited in Table S2 (ESI†), the alter RSD was less than 0.55%, and the recoveries revealed a change in the range from 90% to 103%. The result illustrated that DTFN can serve as an excellent fluorescent nanoprobe for GSH detection in blood serum.
Based on the above admirable features of DTFN to detect GSH, the ability of DTFN to image endogenous GSH in the lysosomes of FR-positive cancer cells was further evaluated. Before imaging, standard MTT assays were utilized to appraise the cytotoxicity of DTFN, as presented in Fig. S16 (ESI†). The results indicated that DTFN had low cytotoxicity towards HeLa cells.
First, to confirm the cellular targeting ability of DTFN, FA-positive HeLa cells and FA-negative A549 cells were selected and co-cultured with DTFN, respectively. As can be seen from Fig. 2, the HeLa cells displayed stronger green fluorescence than A549 cells, demonstrating that DTFN had excellent targeting ability for FR-positive cells. It has been reported that the specific uptake of FA-modified nanoparticles by HeLa cells was proceeded via a FA-receptor-mediated endocytic process.47,55–57
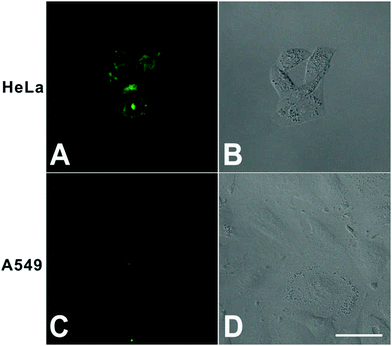 | ||
| Fig. 2 Confocal fluorescence microscopy images of HeLa (A and B) and A549 cells (C and D) treated with DTFN (0.12 mg mL−1). Scale bar: 40 μm. | ||
To further confirm this cellular uptake mechanism, the HeLa cells were incubated with DTFN in FA-containing and FA-free culture medium, respectively (Fig. S17, ESI†). When the HeLa cells were treated with DTFN in the FA-free culture medium, a bright green fluorescence was seen, whereas the HeLa cells treated with DTFN in the FA-containing culture medium depicted a weak green fluorescence. The results confirmed the important role of FA in guiding DTFN to FR-positive cells.
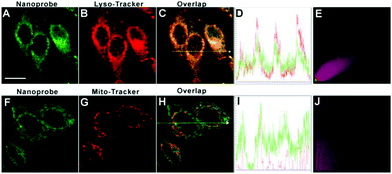
It has been well-documented that nanoprobes often accumulated in lysosomes via the interplay of size and surface charge: (1) nanostructure could be taken up by cells by the energy-dependent endocytosis pathway whose destination is lysosomes;65,66 (2) the positively charged amino moiety enables the nanoparticles to enter into lysosomes.45,58,67,68 To inspect the subcellular targeting ability of DTFN, HeLa cells pre-treated with DTFN were co-incubated with Lyso-Tracker Red and Mito-Tracker Red, respectively. As displayed in Fig. 3, the green fluorescence of DTFN merged well with the red fluorescence of Lyso-Tracker Red (Pearson's correlation coefficient: 0.89). In contrast, the poor colocalization with Mito-Tracker Red (Pearson's correlation coefficient: 0.32) was observed. The above results (High Pearson's correlation coefficient) clearly demonstrated that DTFN can specially accumulate in the lysosomes via the interplay of surface charge and size.
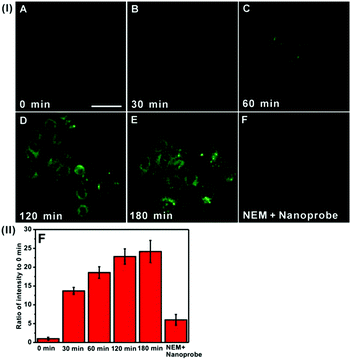
Next, we sought to evaluate the feasibility of DTFN for real-time monitoring endogenous GSH in the lysosomes of HeLa cells (Fig. 4). An obvious green fluorescence enhancement can be visualized with increasing incubation time (Fig. 4B–E), suggesting that the high capacity of DTFN for imaging endogenous GSH in real time. To confirm the specificity of DTFN for intracellular GSH, the HeLa cells were pretreated with the effective GSH inhibitor (N-ethylmaleimide, NEM) and then incubated with DTFN (Fig. 4F). The green fluorescence of cells was remarkably suppressed, confirming that the green fluorescence enhancement of HeLa cells was indeed induced by the endogenous GSH. Apparently, the above results verified that DTFN can be served as an excellent tool to selectively monitor endogenous GSH in the lysosomes of HeLa cells.
Conclusions
In summary, a novel dual-targeting fluorescence nanoprobe (DTFN) was rationally synthesized, and we have demonstrated its excellent capability for efficiently detecting endogenous GSH in the lysosomes of FR-positive HeLa cells. This nanoprobe displayed several advantages over some of the previously reported GSH probes (Table S3, ESI†): (1) the utilization of photostable AIE fluorogen can effectively avoid the aggregation-caused quenching (ACQ) phenomenon of traditional organic small molecule-based GSH probes; (2) the introduction of PEG chains could greatly enhance the water dispersibility and biocompatibility of nanoprobes, while most of the traditional organic small molecule-based GSH probes are usually carried out in organic-aqueous media; (3) by virtue of the dual-targeting strategy, the nanoprobe (DTFN) can successfully achieve the efficient GSH detection in the lysosome of cancer cells. To the best of our knowledge, this is the first study to detect intracellular GSH through a dual-targeting nanoprobe. This methodology provided a new route to the rational design of novel dual-targeting nanoprobes for efficient biomarkers detection in the subcellular organelle of cancer cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support by NSFC (51603067, 51773056, 51873058, 21705040), Hunan Provincial Natural Science Foundation of China (2020JJ3021, 2018JJ3143), China Postdoctoral Science Foundation (2018T110824), Scientific Research Fund of Hunan Provincial Education Department (Project no. 19B204), Open Project Program of State Key Laboratory of Chemo/Biosensing and Chemometrics (2018011), Open Fund of Hunan Provincial Key Laboratory of Advanced Materials for New Energy Storage and Conversion (2018TP1037-202003) and the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology, 2019-skllmd-09).Notes and references
- X. Zhang, J. Wang, X. Li and D. Wang, Cancer Lett., 2018, 439, 39–46 CrossRef CAS PubMed.
- P. Saftig and A. Haas, Nat. Cell Biol., 2016, 18, 1025–1027 CrossRef CAS PubMed.
- J. M. Estrela, A. Ortega and E. Obrador, Crit. Rev. Clin. Lab. Sci., 2008, 43, 143–181 CrossRef PubMed.
- G. Pani, T. Galeotti and P. Chiarugi, Cancer Metastasis Rev., 2010, 29, 351–378 CrossRef CAS PubMed.
- T. Li, C. Yin, J. Chao, W. Zhang and F. Huo, Sens. Actuators, B, 2020, 304, 127431 CrossRef.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X.-P. He and T. D. James,, Acc. Chem. Res., 2019, 529, 2582–2597 CrossRef PubMed.
- S. Singha, Y. W. Jun, S. Sarkar and K. H. Ahn, Acc. Chem. Res., 2019, 52, 2571–2581 CrossRef CAS PubMed.
- Y. Yue, F. Huo, X. Pei, Y. Wang and C. Yin, Anal. Chem., 2020, 92, 6598–6603 CrossRef CAS PubMed.
- J. Zhang, X. Chai, X. P. He, H. J. Kim, J. Yoon and H. Tian, Chem. Soc. Rev., 2018, 48, 683–722 RSC.
- T. Zhou, Y. Yang, K. Zhou, M. Jin, M. Han, W. Li and C. Yin, Sens. Actuators, B, 2019, 301, 127116 CrossRef CAS.
- L. He, B. Dong, Y. Liu and W. Lin,, Chem. Soc. Rev., 2016, 45, 6449–6461 RSC.
- W. Zhang, F. Huo, F. Cheng and C. Yin,, J. Am. Chem. Soc., 2020, 142, 6324–6331 CrossRef CAS PubMed.
- V. S. Lin, W. Chen, M. Xian and C. J. Chang, Chem. Soc. Rev., 2015, 44, 4596–4618 RSC.
- P. Zhang, H. Wang, Y. Hong, M. Yu, R. Zeng, Y. Long and J. Chen, Biosens. Bioelectron., 2018, 99, 318–324 CrossRef CAS PubMed.
- W. Zhang, F. Huo, Y. Yue, Y. Zhang, J. Chao, F. Cheng and C. Yin, J. Am. Chem. Soc., 2020, 142, 3262–3268 CrossRef CAS PubMed.
- S. Yang, J. Jiang, A. Zhou, Y. Zhou, W. Ye, D. S. Cao and R. Yang, Anal. Chem., 2020, 92, 7194–7199 CrossRef CAS PubMed.
- H. Li, H. Lin, W. Lv, P. Gai and F. Li, Biosens. Bioelectron., 2020, 165, 112336 CrossRef CAS PubMed.
- T. Li, C. Yin, J. Chao, W. Zhang and F. Huo, Sens. Actuators, B, 2020, 305, 127336 CrossRef.
- Y. Zhou, S. Yang, J. Guo, H. Dong, K. Yin, W. T. Huang and R. Yang, Anal. Chem., 2020, 92, 5787–5794 CrossRef CAS PubMed.
- W. Zhang, F. Huo and C. Yin, Org. Lett., 2019, 21(13), 5277–5280 CrossRef CAS PubMed.
- X. Wu, W. Shi, X. Li and H. Ma, Acc. Chem. Res., 2019, 52, 1892–1904 CrossRef CAS PubMed.
- P. Zhang, H. Wang, D. Zhang, X. Zeng, R. Zeng, L. Xiao, H. Tao, Y. Long, P. Yi and J. Chen, Sens. Actuators, B, 2018, 255, 2223–2231 CrossRef CAS.
- H. Wei, R. Zeng, S. Wang, C.-H. Zhang, S. Chen, P. Zhang and J. Chen, Mater. Chem. Front., 2020, 4, 862–868 RSC.
- Y. Yang, T. Zhou, M. Jin, K. Zhou, D. Liu, X. Li, F. Huo, W. Li and C. Yin, J. Am. Chem. Soc., 2020, 142, 1614–1620 CrossRef CAS PubMed.
- J. Ren, P. Zhang, H. Liu, C. Zhang, Y. Gao, J. Cui and J. Chen, Sens. Actuators, B, 2020, 304, 127299 CrossRef.
- H. Chen, Y. Tang and W. Lin, TrAC, Trends Anal. Chem., 2016, 76, 166–181 CrossRef CAS.
- J. Liu, Y.-Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574–577 CrossRef CAS PubMed.
- L.-Y. Niu, Y.-Z. Chen, H.-R. Zheng, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Soc. Rev., 2015, 44, 6143–6160 RSC.
- Z. Yuan, L. Gui, J. Zheng, Y. Chen, S. Qu, Y. Shen, F. Wang, M. Er, Y. Gu and H. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 30994–31007 CrossRef CAS PubMed.
- M. H. Lee, J. H. Han, P.-S. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316–1322 CrossRef CAS PubMed.
- R. Guo, F. Huang, B. Zhang, Y. Yan, J. Che, Y. Jin, Y. Zhuang, R. Dong, Y. Li, B. Tan, R. Song, Y. Hu, X. Dong, X. Li and N. Lin, Theranostics, 2019, 9, 3515–3525 CrossRef CAS PubMed.
- D. Feng, Y. Song, W. Shi, X. Li and H. Ma, Anal. Chem., 2013, 85, 6530–6535 CrossRef CAS PubMed.
- S. Zang, X. Kong, J. Cui, S. Su, W. Shu, J. Jing and X. Zhang, J. Mater. Chem. B, 2020, 8, 2660–2665 RSC.
- Q. Gao, W. Zhang, B. Song, R. Zhang, W. Guo and J. Yuan, Anal. Chem., 2017, 89, 4517–4524 CrossRef CAS PubMed.
- M. Cao, H. Chen, D. Chen, Z. Xu, S. H. Liu, X. Chen and J. Yin, Chem. Commun., 2016, 52, 721–724 RSC.
- H. Zhao, X. Wen, W. Li, Y. Li and C. Yin, J. Mater. Chem. B, 2019, 7, 2169–2176 RSC.
- Z. Xu, X. Huang, X. Han, D. Wu, B. Zhang, Y. Tan, M. Cao, S. H. Liu, J. Yin and J. Yoon, Chemistry, 2018, 4, 1609–1628 CrossRef CAS.
- S.-Y. Lim, K.-H. Hong, D. I. Kim, H. Kwon and H.-J. Kim, J. Am. Chem. Soc., 2014, 136, 7018–7025 CrossRef CAS PubMed.
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC.
- Q. Miao, C. Xie, X. Zhen, Y. Lyu, H. Duan, X. Liu, J. V. Jokerst and K. Pu, Nat. Biotechnol., 2017, 35, 1102–1110 CrossRef CAS PubMed.
- L. Bau, P. Tecilla and F. Mancin, Nanoscale, 2011, 3, 121–133 RSC.
- L. Feng, C. Zhu, H. Yuan, L. Liu, F. Lv and S. Wang, Chem. Soc. Rev., 2013, 42, 6620–6633 RSC.
- X. Huang, J. Song, B. C. Yung, X. Huang, Y. Xiong and X. Chen, Chem. Soc. Rev., 2018, 47, 2873–2920 RSC.
- J. Hu and S. Liu, Acc. Chem. Res., 2014, 47, 2084–2095 CrossRef CAS PubMed.
- H. Wang, P. Zhang, Y. Hong, B. Zhao, P. Yi and J. Chen, Polym. Chem., 2017, 8, 5795–5802 RSC.
- J. Yu, Y. Rong, C. T. Kuo, X. H. Zhou and D. T. Chiu, Anal. Chem., 2016, 89, 42–56 CrossRef PubMed.
- J. Xu, F. Zeng, H. Wu, C. Yu and S. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 9287–9296 CrossRef CAS.
- S. Chen, J. Fan, W. Qiu, F. Liu, G. Yan, X. Zeng and X. Zhang, J. Mater. Chem. B, 2018, 6, 1543–1551 RSC.
- H. Chen, Y. Chen, H. Yang, W. Xu, M. Zhang, Y. Ma, S. Achilefu and Y. Gu, Polym. Chem., 2014, 5, 4734–4746 RSC.
- J. Wang, Y. Yang, Y. Zhang, M. Huang, Z. Zhou, W. Luo, J. Tang, J. Wang, Q. Xiao, H. Chen, Y. Cai, X. Sun, Y. Wang and Y. Ke, Adv. Funct. Mater., 2016, 26, 7873–7885 CrossRef CAS.
- B. Y. Liu, X. Y. He, C. Xu, L. Xu, S. L. Ai, S. X. Cheng and R. X. Zhuo, Biomacromolecules, 2018, 19, 2957–2968 CrossRef CAS PubMed.
- Y. Zhong, F. Meng, C. Deng and Z. Zhong, Biomacromolecules, 2014, 15, 1955–1969 CrossRef CAS PubMed.
- S. Fu, M. Liang, Y. Wang, L. Cui, C. Gao, X. Chu, Q. Liu, Y. Feng, W. Gong, M. Yang, Z. Li, C. Yang, X. Xie, Y. Yang and C. Gao, ACS Appl. Mater. Interfaces, 2019, 11, 1841–1854 CrossRef CAS.
- L. Dai, R. Cai, M. Li, Z. Luo, Y. Yu, W. Chen, X. Shen, Y. Pei, X. Zhao and K. Cai, Chem. Mater., 2017, 29, 6976–6992 CrossRef CAS.
- J. Xu, F. Zeng, H. Wu, C. Hu and S. Wu, Biomacromolecules, 2014, 15, 4249–4259 CrossRef CAS PubMed.
- J. Fan, G. Fang, F. Zeng, X. Wang and S. Wu, Small, 2013, 9, 613–621 CrossRef CAS PubMed.
- J. Fan, F. Zeng, S. Wu and X. Wang, Biomacromolecules, 2012, 13, 4126–4137 CrossRef CAS PubMed.
- J. Chen, Y. Tang, H. Wang, P. Zhang, Y. Li and J. Jiang, J. Colloid Interface Sci., 2016, 484, 298–307 CrossRef CAS PubMed.
- W. Zhong, X. Zeng, J. Chen, Y. Hong, L. Xiao and P. Zhang, Polym. Chem., 2017, 8, 4849–4855 RSC.
- H. M. Meng, Z. Jin, Y. Lv, C. Yang, X. B. Zhang, W. Tan and R. Q. Yu, Anal. Chem., 2014, 86, 12321–12326 CrossRef CAS PubMed.
- R. Deng, X. Xie, M. Vendrell, Y. T. Chang and X. Liu, J. Am. Chem. Soc., 2011, 133, 20168–20171 CrossRef CAS PubMed.
- F. Yu, P. Li, B. Wang and K. Han, J. Am. Chem. Soc., 2013, 135, 7674–7680 CrossRef CAS PubMed.
- Q. Wang, Y. Zhang, X. Wang, Y. Wu, C. Dong and S. Shuang, Analyst, 2019, 144, 1988–1994 RSC.
- Q. Y. Cai, J. Li, J. Ge, L. Zhang, Y. L. Hu, Z. H. Li and L. B. Qu, Biosens. Bioelectron., 2015, 72, 31–36 CrossRef CAS PubMed.
- X. Ma, Y. Wu, S. Jin, Y. Tian, X. Zhang, Y. Zhao, L. Yu and X.-J. Liang, ACS Nano, 2011, 5, 8629–8639 CrossRef CAS PubMed.
- D. Huo, Q. Chen, J. Xue, S. Shen and Y. Xia, Adv. Mater., 2017, 29, 1703702 CrossRef PubMed.
- Y. Jiang, S. Huo, T. Mizuhara, R. Das., Y. Lee, S. Hou, D. F. Moyano, B. Duncan, X.-J. Liang and V. M. Rotello, ACS Nano, 2015, 9, 9986–9993 CrossRef CAS PubMed.
- J. Mosquera, I. García and L. M. Liz-Marzán, Acc. Chem. Res., 2018, 51, 2305–2313 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00124d |
| This journal is © The Royal Society of Chemistry 2020 |