Open Access Article
Open Access ArticleOxygen, nitrogen co-doped molybdenum disulphide nanoflowers for an excellent antifungal activity†
Parbati
Basu
a,
Khushi
Mukherjee
b,
Sudipta
Khamrui
c,
Subharaj
Mukherjee
d,
Maudud
Ahmed
d,
Krishnendu
Acharya
b,
Debamalya
Banerjee
 c,
Padinharu M. G.
Nambissan
c,
Padinharu M. G.
Nambissan
 d and
Kuntal
Chatterjee
d and
Kuntal
Chatterjee
 *a
*a
aDepartment of Physics, Vidyasagar University, Midnapore-721102, India. E-mail: kuntal@mail.vidyasagar.ac.in
bMolecular and Applied Mycology and Plant Pathology Laboratory, Department of Botany, University of Calcutta, Kolkata-700019, India
cDepartment of Physics, IIT Kharagpur, Kharagpur-721302, India
dApplied Nuclear Physics Division, Saha Institute of Nuclear Physics, Bidhannagar, Kolkata-700064, India
First published on 16th July 2020
Abstract
The engineering of pristine low dimensional materials towards society-needed functionalities is the driving force for cultivation the field of nano–bio research. Herein, the molybdenum disulphide structure has been ornamented with nitrogen as well as oxygen atoms by a facile chemical route. This O,N co-doped MoS2 nanoflower sample along with only-N-doped MoS2 samples were investigated structurally and chemically to ascertain the successful attachment of the dopant atoms and its associated changes in the structure. While positron annihilation spectroscopy demonstrates detailed defect pictures of these two variants with not much of a difference, the electron paramagnetic resonance displays the adequacy of Mo5+ defect states in O,N co-doped MoS2 compared to the only-N-doped MoS2 sample. Moreover, this O,N co-doped MoS2 exhibits outstanding antifungal activities towards two harmful fungal pathogens, namely, Alternaria alternata and Fusarium oxysporum, in dark as well as in light. MoS2, which otherwise showed no antifungal growth inhibition whatsoever, can thus be promoted to get exalted antifungal performance by gradual incorporation of nitrogen and oxygen atoms within the lattice of MoS2. This study establishes a new pathway to design better candidates for antifungal activity with lesser chances of antimicrobial resistance through suitable elemental engineering and is a prime need for our society to cope with the growing unforeseen dangers.
1. Introduction
In the wake of the vast catastrophe foisted on by the potato late blight-led Irish famine that went on to change the course of European history,1 it became truly imperative to develop rigorous pre-emptive measures to combat such invasive fungal infections in times to come. However, over the past centuries, the numerals of fungi or fungus-like oomycetes causing virulent diseases have only proliferated to pose a grave threat to the ecosystem. If not checked in time, this would not only put the ecological homeostasis at stake but also potentially disrupt the food security, consequentially leading to a debilitated agricultural output. Furthermore, the efficacy of the presently available antifungal remedies in humans, namely, polyenes, azoles, flucytosine, and echinocandins has plateaued out2 and the incidences of invasive fungal infections in immuno-compromised human population are incessantly on the rise.3 Ironically, the presence of several limitations, such as off-target toxicity, adverse interactions with other drugs, and the emergence of antifungal resistant strains have rendered these existent antifungals delimited in combating refractory and invasive fungal infections. The use of chemical-based pesticides, on the other hand, in the management of pre- and post-harvest decay and diseases in crops has been a long-standing issue of concern in the light of environmental pollution and safety in consuming foods ensuing out of toxic residuals of these fungicides.4 Therefore, it is essential to develop new environment-friendly antifungal nanomaterials with lower levels of toxicity and less chances of microbial resistance. In this matter, nanomaterial-based antifungals have evolved to be an exceptional alternative.5 The repertoire of nanomaterials explored so far in combating microbial invasions comprises of plasmonic nanoparticles,6 metal oxides,7,8 graphene oxide,9 graphene-based derivatives,10,11 and various hetero-structured nanocomposites.12–15 Of late, inspired by the grand success of graphene and graphene-based nanomaterials in antimicrobial activity, layered 2D materials, particularly molybdenum disulphide (MoS2), have started to garner lot of interest in this field. However, till now, reports on the antifungal activities of MoS2 are strikingly very few in number.Owing to the innumerable novel and intriguing properties arising due to quantum confinement in the third dimension, MoS2, a layered transition metal dichalcogenide, has found extensive applications in transistors,16 optoelectronic devices,17 spintronics,18 sensors,19 catalysts,20 photodetectors,21 energy storage devices,22 biomedicine,23etc. In MoS2, a layer of Mo atoms is sandwiched between two layers of S atoms held by strong covalent bonding.24 However, due to the weak interlayer van der Waals force, mono- or few-layered MoS2 nanosheets with large specific surface area can be easily extracted from the bulk analogues by mechanical or chemical exfoliation methods.24 In addition, fluorescence or quenching of the fluorescence properties, which are entirely dependent on the thickness of the layers and the crystal structure,25,26 strong near-infrared region absorbance, and high photothermal quenching efficiency,27 have enabled the use of MoS2 in various biomedical applications including bio-sensing, bio-imaging, cancer therapy, drug delivery, antibacterial wound healing, and tissue regeneration.28,29 In spite of all these bio-related applications, the antifungal activities of 2D MoS2 have remained largely unexplored till now. Although pristine bulk MoS2 has been hardly reported to exhibit any microbial inhibition, by controlling the thickness of the layers, lateral size, and crystal structure, the desired antimicrobial properties may be enforced within MoS2. Liu et al. demonstrated the photocatalytic bacterial disinfection of water by few-layered vertically aligned MoS2 nanofilms.30 Recently, Xu et al. showed that nanoflowers of MoS2 exhibited better antibacterial capabilities than the MoS2 nanosheets.31 Surface functionalization with hydrophobic ligands,32 antimicrobial peptides,33 and loaded antibiotics34 have been found to induce antimicrobial properties within the MoS2 nanosheets as well. Another fruitful strategy to impose antimicrobial behavior within the MoS2 nanosheets is to combine them with other antimicrobial nanomaterials such as silver and graphene oxide.35 Zhang et al. showed that chitosan and silver nanoparticle-modified MoS2 exhibited effectively large antifungal inhibition of Saccharomyces uvarum and Aspergillus niger.36 However, in all these cases, the bactericidal activities were observed to be engendered by light-dependent stimulations, which would therefore wholly restrain the scope of application of these antimicrobials for disinfection in implantable medical devices. In our previous work,37 we have shown that the defect sites, which are relatively abundant in MoS2, can be advantageously manipulated through nitrogen doping to make them suitable for the generation of reactive oxygen species (ROS) in dark as well, thereby invoking antifungal properties within it. Therefore, it was found that the advantageous design of the material in an appropriate way holds the key to attain success in developing powerful antifungals from MoS2 nanostructures; this is a relatively new and interesting field of study that requires intensive research work.
Here, in this work, we designed MoS2 nanostructures through suitable elemental doping and demonstrate how doping helps in significantly inducing high antifungal abilities in the MoS2 nanostructures, which would otherwise have no inhibitory effect on the fungal growth of phytopathogens. Moreover, we shall explain how the efficacy of the induced antifungal activity evolves on being co-doped with elements of differing electronegativities. Doping has always been a useful strategy to tune and reform functionalities such as electronic, magnetic, and catalytic.38–40 Interestingly, oxygen incorporation has been previously reported to have enhanced the catalytic performances in electrocatalytic hydrogen generation,39,40 supercapacitor,41etc., but there are no reports on the doping by oxygen to achieve antifungal performances in MoS2 so far. Herein, we successfully doped oxygen in MoS2 in addition to N-doping through the facile hydrothermal method and studied its antifungal activities on Alternaria alternata and Fusarium oxysporum as version representatives of harmful fungal pathogens. Both these species have devastating effects not just on agricultural plants, leading to huge losses during the pre and post-harvest phases but also immunity-compromised humans and animals as well. Hence, the need to develop effectively potent antifungals to combat these fungal pathogens has grown to be an absolute necessity. Further, to get a comprehensive insight into the origin of the antifungal activities in these doped samples, various characterisation techniques were adopted and a possible mechanism and pathway leading to the fungal deaths has also been proposed.
2. Experimental section
2.1 Materials
All the chemicals and reagents were of analytical grade and purchased from Sigma Aldrich. They were used without any further purification.2.2 Synthesis of nitrogen-doped MoS2 and oxygen–nitrogen co-doped MoS2
Here, in this work, nitrogen-doped molybdenum disulphide and oxygen–nitrogen co-doped MoS2 nanostructures, henceforth referred to as NMS and ONMS, respectively, were successfully synthesized through an optimised one-step hydrothermal method. On the other hand, bulk MoS2, henceforth referred to as MS in the text, of analytical grade was procured commercially from Sigma Aldrich. In order to prepare nitrogen-doped MoS2 (NMS), 0.5038 g of molybdenum trioxide (MoO3) dissolved in 15 mL of DI water was added drop-wise to 0.8 M aqueous solution of thiourea (CSN2H4) and stirred vigorously on a magnetic stirrer for an hour. The resultant bluish white solution was then transferred to an autoclave of capacity 75 mL and heated in an oven at 180 °C for 48 hours. The black product so obtained was washed several times with de-ionised water and eventually dried at 60 °C to obtain the nitrogen-doped MoS2 powder (NMS). Further, oxygen–nitrogen co-doped MoS2 (ONMS) was prepared via a similar hydrothermal procedure, wherein the same precursor ratio and reaction temperature were maintained but the time of hydrothermal reaction was reduced to 18 hours.2.3 Characterisation
In order to obtain the X-ray diffraction patterns, the synthesized powder samples were scanned in the 2θ range of 3°–80° with a step size of 0.02° in a Rigaku Miniflex X-ray diffractometer having CuKα radiation source of wavelength 1.5418 Å. A scanning electron microscope from Zeiss was used to analyse the morphology of the as-synthesized samples. Further, the transmission electron microscopy analysis of the obtained samples was done on a FEI-made Technai G2 20 Twin high resolution transmission electron microscope. The surface area measurements (BET) and pore size analysis was performed on the AutosorbiQ station from Quantachrome instruments. The Renishaw inVia Raman microscope was utilised to acquire the Raman spectra with an excitation wavelength of 532 nm at the room temperature. The X-ray photoelectron spectroscopy studies were recorded using a ULVAC-PHI5000 Versa Probe II spectrometer equipped with an Al Kα radiation source having 1486.6 eV of photon energy. The X-band electron paramagnetic resonance signals were recorded on the Bruker-made ELEXSYS E580 pulsed electron paramagnetic resonance spectrometer at room temperature. The microwave power for the measurements were fixed at 15 mW. The details of positron annihilation spectroscopy (PAS) and coincidence Doppler broadening spectroscopy (CDBS) experiments are provided in the ESI† (Section S1.1).2.4 Antifungal studies
3. Results and discussion
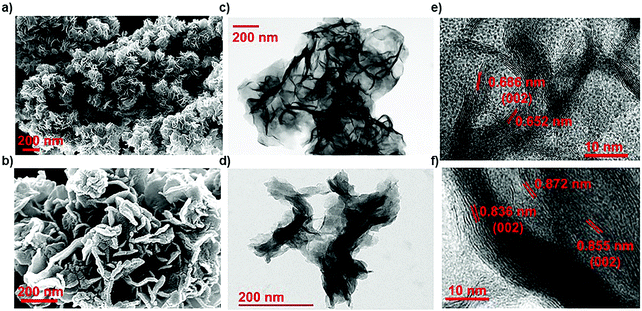
Here, in this work, the incorporation of only nitrogen atoms in the case of NMS and both oxygen and nitrogen in the case of ONMS as dopants within the lattice was optimised and achieved through sustainable control on the time over which the reaction took place. NH3 evolution as a by-product in the thermal decomposition of thiourea accounts for the nitrogen atoms being doped within the MoS2 lattice, which according to our previous study, are most likely to occupy the S-sites in the Mo-rich limit.37 However, in order to ensure the incorporation of oxygen into the MoS2 lattice in addition to the nitrogen atoms, the reaction time had to be substantially brought down to 18 hours. The reduced reaction time effectively rendered the reaction process insufficient for the complete conversion of MoO3 to MoS2. Hence, traces of unsaturated Mo–O bonds, inherited from the MoO3 precursor, remained within the lattice of MoS2 to ensure the presence of oxygen atoms within the MoS2 lattice. Previously, in a similar kind of synthetic procedure, Xie et al. had incorporated oxygen in MoS2 nanosheets by controlling the crystallisation process through temperature variation in order to achieve enhanced electro-catalytic activity in the hydrogen evolution reaction.40 Besides, in earlier works, surface oxidation achieved through various other methods such as exposure to high energy RF oxygen plasma,42 remote plasma,43 1.5–5 eV atomic oxygen flux,44 UV-ozone exposure,45 and annealing in a high temperature oxygen environment,46 were used. These are rather harsh methods and resulted in non-uniform lateral oxidation and in-depth lattice etching. In contrast, the strategy adopted in this present work is a far more simplistic approach, resulting in a high percentage of oxygen doping.In order to determine the morphology and microstructure of the as-grown doped MoS2 samples, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were conducted. The SEM images, as shown in Fig. 1(a) and (b), reveal almost similar kinds of flower-like morphology for both NMS and ONMS. However, in the case of ONMS, the MoS2 nanosheets, which assembled themselves into a flower-like motif, are apparently seen to be considerably thickened and also have surface roughness. Further, in ONMS, the edges of the nanosheets are obscure, suggesting insufficient crystallisation due to reduced reaction time. On the contrary, the NMS sheets have relatively sharper edges and reveal better crystallinity. The high magnification TEM images [Fig. 1(c) and (d)] further indicate the assemblage of nanosheets in a flower-like manner, as obtained from scanning electron microscopy. The high-resolution transmission electron microscopy (HRTEM) images reveal an enlarged d-spacing of ∼0.855 nm ONMS compared to that of 0.66 nm [Fig. 1(e) and (f)]. In congruence with the earlier studies,39–41 this enlargement in the interplanar d-spacing can be attributed to the incorporation of oxygen in the MoS2 lattice. However, the d002 value calculated from the fringes of NMS agrees well with the reported values of 2H-MoS2.37 In addition to this, X-ray diffraction (XRD) studies were carried out on the synthesized samples in order to figure out the crystal structure. The XRD pattern for NMS, as shown in Fig. 2(a), matches well with the data of 2H-MoS2 (JCPDS card no. 87-2416). The interplanar spacing (d002) calculated from the XRD peak value agrees well with those calculated from the HRTEM fringes. However, as evident from Fig. 2(a), two new peaks at 10.28° and 16.87° were seen to emerge for ONMS. The d-spacing calculated for these two peaks is 0.861 nm and 0.524 nm, respectively. This diploidic relation corresponding to the interplanar spacing unambiguously suggests that a new lamellar structure with an expanded interplanar spacing has been formed. This possibly accounts for the thickening of the nanosheets in ONMS as observed from the SEM images. The simulated XRD pattern for oxygen incorporated MoS2 nanosheets taken from Xie et al. is inserted in Fig. 2(a) for comparison and is seen to match with the XRD peaks of ONMS in the low angle region.40 Nonetheless, the XRD peaks in ONMS corresponding to the (102) and (110) planes match with those of 2H-MoS2.37 The surface area and porosity of the synthesized samples were ascertained by the adsorption–desorption studies of nitrogen. The isotherm plots for ONMS and NMS are shown in Fig. S1 (ESI†) and suggest the presence of mesopores within the samples. Applying Brunauer–Emmett–Teller (BET) analysis, the surface areas for ONMS and NMS were calculated to be 6.507 m2 g−1 and 6.484 m2 g−1, respectively. Further, the average pore radius in ONMS was 38.468 Å with a total pore volume of 1.252 × 10−2 cc g−1 for the pores of radius less than 144.9 nm (P/P0 = 0.993). In contrast, NMS showed a marginally increased average pore radius of 40.78 Å with a total pore volume of 1.332 × 10−2 cc g−1 for the pores of radius less than 210.4 nm (P/P0 = 0.995).
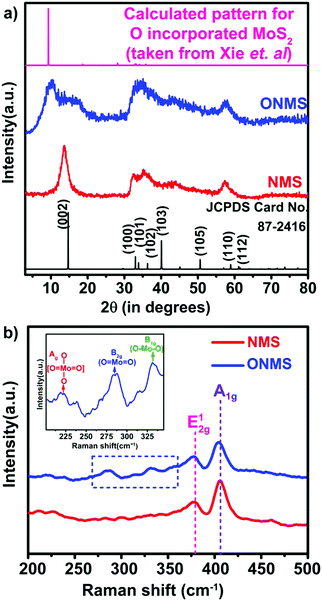 | ||
| Fig. 2 (a) X-ray diffraction patterns NMS and ONMS. The standard pattern for commercially procured 2H-MoS2 and the calculated pattern for oxygen-incorporated MoS2 lattice (taken from Xie et al.)40 is given as the reference. (b) Raman spectra for NMS and ONMS. The dotted blue rectangle emphasizes the development of the Mo–O bond in ONMS. The inset shows the presence of three Raman peaks assigned to different Mo–O bond formations in ONMS under zoomed condition. | ||
Raman spectroscopy was further employed to look into the modes of vibration in the samples in greater details. In Fig. 2(b), the obtained Raman spectra for NMS and ONMS are shown. In the 2H representative of bulk MoS2, the two Raman active modes are the E12g mode resulting from the oppositely directed in-plane vibration of the Mo and S atoms and the A1g mode as a consequent of their out-of-plane opposite vibrations.47 The E12g and A1g modes occur nearly at 382 cm−1 and 407 cm−1 in the bulk form of MoS2.47 However, in the case of both ONMS and NMS, the E12g mode is red-shifted by ∼4.5 cm−1 in comparison to the reported values of 6-layered MoS2.47 This red shift possibly ensues from the lattice distortion invoked by oxygen and nitrogen doping in the samples. This is further reaffirmed by the disorder-prompted symmetry breaking of the E12g mode, causing it to split into the 1+2g and E1−2g components,37,48 as shown in Fig. S2 (ESI†). Meanwhile, the A1g Raman mode is red-shifted in ONMS by almost 3 cm−1 relative to the 6-layered MoS2, which is more pronounced than the ∼1 cm−1 red-shift in the case of NMS. Moreover, the emergence of Raman peaks at 284.32 cm−1, 331.18 cm−1, and 218.75 cm−1, assigned to the B2g, B1g, and Ag modes of vibration for the Mo–O bonds, reconfirms the successful doping of oxygen in ONMS.40,49
In order to get further insight about the chemical constitution of the as-synthesized samples, X-ray photoelectron spectroscopy (XPS) was performed on the doped MoS2 samples. The multi-component core level XPS spectra was deconvoluted with the help of mixed Gaussian–Lorentzian product function alongside a Shirley background correction using the CASAXPS software. In NMS, the deconvolution of the core Mo 3d region resulted in two doublets, one between ∼228.6 eV and 231.74 eV, and the other between ∼228.9 eV and 232.04 eV, as seen from Fig. 3(a). The lower binding energy doublet corresponds to the Mo 3d5/2, and Mo 3d3/2 core levels in MoS2, i.e., Mo4+ and S2− charge states arising out of S–Mo charge transfer.48,50 The corresponding peaks in the core level S 2p spectra for the S2− charge state are seen at 161.61 eV and 162.74 eV. The other doublet in the Mo 3d region, at binding energies higher than the Mo–S peak by ∼0.3 eV, which may be attributed to the formation of Mo–N bonds in agreement with the previous reports.48 This is further affirmed by the presence of the peak at 397.59 eV in the N 1s region [Fig. 3(c)], which essentially signifies Mo–N bond formation.37,48,51 Also, the N 1s region is partially overlapped by the core level Mo 3p3/2 region and the peak at 394.47 eV originates from the Mo–S bonds in MoS2.37,48 The remaining peak at ∼401 eV arises because of the strongly chemisorbed ammonia.37,51 A small hump near 232.95 eV in the Mo 3d region of NMS may be ascribed to superficial oxidation of the synthesized NMS sample instead of any Mo–O bond formation. A similar peak at ∼233.4 eV is present in the Mo 3d region of ONMS as well. The absence of any Mo–O peaks in the O 1s region of NMS further substantiates our claim of surface oxidation. The peaks at 531.26 eV, 532.3 eV, and 533.77 eV in the O 1s region of NMS, as shown in Fig. S3(b) (ESI†), are primarily due to oxygen in the –OH radical (possibly physisorbed H2O), O2 adsorbed from the atmosphere, and physisorbed/chemisorbed H2O at the surface respectively.52–54 Further, the peaks at 226.17 eV and 224.87 eV in the overlapped S 2s region arise because of Mo–S bonding in MoS2 and the cluster complexes of Mo and S, respectively.55 On the other hand, in ONMS, the doublets in the Mo 3d region arising out of Mo–S and Mo–N bond formation are shifted down to lower binding energies, as is revealed from Fig. 3(a). The S 2p peaks associated with the S2− state are also downshifted to lower energy values, as shown in Fig. 3(b). The intensity of the doublet arising out of Mo–N bonding, however, decreased for ONMS, suggesting reduced N-doping compared to NMS. In addition to this, another doublet is seen to arise between 229.72 eV and 232.73 eV in Mo 3d region for ONMS. According to earlier reports,56 this doublet emerges because of the incorporation of oxygen in MoS2 to form a MoS2−xOx-like phase with the Mo5+ charge state. Further, the peak at 399.162 eV in the overlapped N 1s region of ONMS [Fig. 3(c)], in addition to the little Mo–N peak at 397.44 eV, emerges due to the introduction of Mo–O bonds.57 Also, the peak at 530.2 eV in the core level of the O 1s region for ONMS reaffirms the presence of Mo–O bonds41 in it, as evident from Fig. S3(a) (ESI†). However, the other peaks at 531.10 eV and 532.06 eV in the O 1s region are due to the –OH radical and adsorbed oxygen. Hence, it can be inferred that in addition to being nitrogen-doped, oxygen has also been successfully incorporated into the MoS2 lattice of ONMS through substitutional doping rather than surface oxidation. The downshift of the doublets in the core level Mo 3d region of ONMS, as mentioned before, may be explained to occur as a result of the Fermi level realignment between MoS2 and MoOx, giving rise to upward band bending.43,58 The peak at 235.29 eV in the Mo 3d region suggests the presence of residual unreacted MoO3 for ONMS.37 The S 2p region for ONMS [Fig. 3(b)] on being deconvoluted resulted primarily into two doublets, out of which, one with its peaks at 161.17 eV and 162.41 eV arises because of the S2− state of MoS2 and the other between 162.86 eV and 164 eV results because of the S22− state, which is normally seen to accompany the Mo5+ charge state.50,59 The quantitative analysis of the survey spectra, as shown in Fig. S4 (ESI†), reveals 15.97% of oxygen doping as well as 4.89% of N-doping in ONMS whereas there is 6.38% nitrogen-doping in NMS.
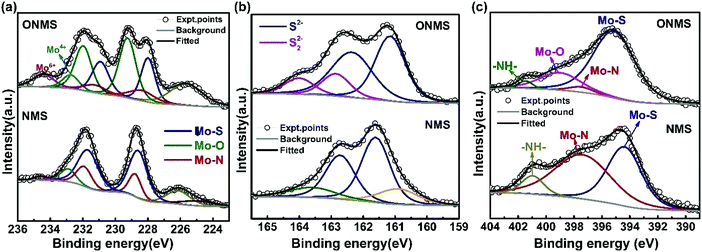 | ||
| Fig. 3 High resolution X-ray photoelectron spectrum from the (a) Mo 3d, (b) S 2p, and (c) N 1s regions of ONMS and NMS. | ||
The X-band electron paramagnetic resonance (EPR) study conducted on the doped MoS2 samples revealed the presence of a weak broad signal at ∼g = 2.00 in both the ONMS and NMS samples, as shown in Fig. 4(a). This signal in the EPR spectrum, according to previous studies, arises due to sulphur-coordinated (thio-Mo5+) defects in MoS2.60 The intensities of the ∼g = 2.00 signal in both ONMS and NMS appear to be almost equal at a glance, indicating that there is not much of a difference in the coordination environment of the sulphur-moderated defect sites or their relative abundance. However, the presence of a peak at g = 1.9364 [Fig. 4(a)] for ONMS makes it different from NMS as far as the oxygen-coordinated (oxo-Mo5+) defect states are concerned. According to earlier reports, the g = 1.9364 line arises because of the hyperfine interaction of the unpaired electrons in the Mo5+ state, which consists of a molybdenyl centre, i.e., a short Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond surrounded by oxygen ligands in distorted octahedral or square-pyramidal symmetry.50,61 Therefore, this strongly evinces the successful doping of oxygen atoms throughout the sample in the case of ONMS.
O bond surrounded by oxygen ligands in distorted octahedral or square-pyramidal symmetry.50,61 Therefore, this strongly evinces the successful doping of oxygen atoms throughout the sample in the case of ONMS.
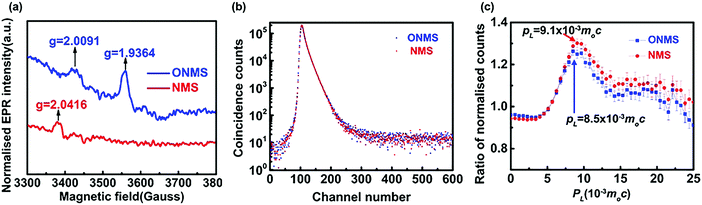 | ||
| Fig. 4 (a) EPR spectra for ONMS and NMS. (b) Peak normalised positron lifetime spectra for ONMS and NMS. (c) Ratio curves generated from the coincidence Doppler broadening spectra of ONMS and NMS. | ||
In order to get a comprehensive insight into the nature of the defect states, as revealed by the EPR spectra, positron annihilation spectroscopy (PALS) was carried out on both ONMS and NMS. The peak-normalised positron lifetime spectra of ONMS and NMS are given in Fig. 4(b). Not much of a difference is observed between the two samples, although the resolved positron lifetimes and intensities (showed in Table 1) are more informative and sharper in detail as far as the sizes and concentrations of the defect structures present in the samples are concerned.
| Sample | τ 1 (ns) | τ 2 (ns) | τ 3 (ns) | I 1 (%) | I 2 (%) | I 3 (%) | τ m (ns) | τ b (ns) | τ 2 − τb (ns) | κ (ns−1) | μ (ns−1) | C (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ONMS | 0.1014 ± 0.0021 | 0.2960 ± 0.0034 | 0.55 | 34.49 ± 0.82 | 52.41 ± 0.63 | 13.10 ± 0.37 | 0.2621 | 0.1848 | 0.1112 | 3.0891 | 0.6 | 5.148 |
| NMS | 0.1033 ± 0.0020 | 0.3047 ± 0.0029 | 0.55 | 33.28 ± 0.72 | 56.52 ± 0.55 | 10.20 ± 0.32 | 0.2627 | 0.1900 | 0.1147 | 3.3648 | 0.6 | 5.608 |
The observation of a third positron(ium) lifetime component τ3 is quite unnatural for materials with relatively higher electron densities like as MoS2 since it normally arises due to the formation of positronium (Ps), which is the metastable bound state of an electron and a positron. It can happen in nanocrystalline materials where large intercrystalline boundaries exist, which are characterised by the significantly reduced electron density compared to that of the bulk. However, in this case, the efforts to analyse the spectra with two lifetime components yielded poor reduced χ2 fit. The three-component analysis also gave scattered values of the third component of lifetime τ3 and relative intensity I3. So, the analyses were done by fixing τ3 to 0.55 ns, which incidentally is the highest possible positron lifetime when it is annihilated from the nanocrystalline surfaces. From the results shown in Table 1, the first conclusion that can be drawn is the existence of two types of structural defects, one arising out of the atomistic defects or crystalline vacancies and the other as porous type defects in the free spaces within the molecular assemblage, as revealed by the BET surface analysis. The positron lifetimes τ2 and τ3 represent these defects and the magnitudes of these lifetimes also indicate the nature and sizes of the defects. For example, the lifetime of the positrons annihilating from the free state (i.e., not trapped in any defect and diffusing freely through the inter-atomic region) is calculated for both the cases from the relation
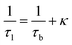 | (1) |
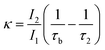 | (2) |
The coincidence Doppler broadening spectra (CDBS), as shown in Fig. 4(c), also give results that are consistent with those obtained from the positron annihilation lifetime measurements. The peak at PL = 9.1 × 10−3m0c that arises from positron annihilation with the 4p electrons of Mo is stronger in the case of NMS when compared to that of ONMS. This again proves that the defect concentration in NMS is slightly on the higher side. The calculated defect concentration, as obtained from the positron lifetime results, for the NMS samples is roughly 10% higher than that of ONMS, which is consistent with the observations of CDBS. Since O2 doping is there in ONMS, the peak of CDBS is slightly shifted towards PL = 8.5 × 10−3m0c, which indirectly hints at the annihilation of positrons with the 2p electrons of O2 atoms (PL = 8.5 × 10−3m0c).
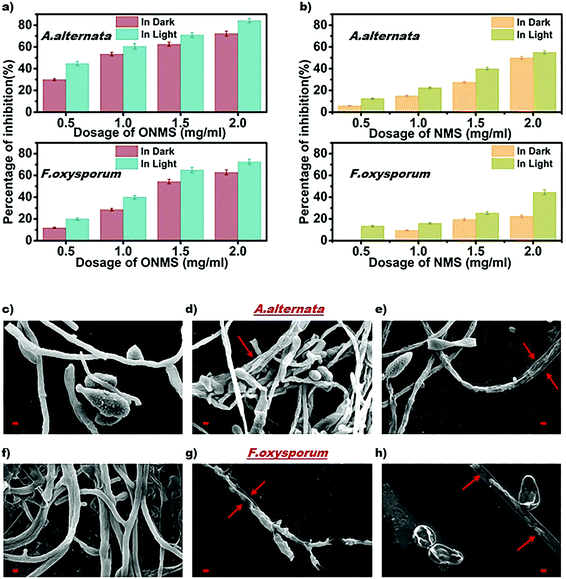
The as-grown NMS and ONMS samples along with pristine MoS2 (MS) were employed to study their antifungal properties on fungal pathogens, namely, Alternari aalternata and Fusarium oxysporum and to understand whether doping with elements of different electronegativities affects the fungal inhibition process. The fungitoxicity of the MoS2 nanostructures, as found from our previous study, are highly dependent on their ROS (reactive oxygen species) generation capacity both in dark and under visible light irradiation.37 The fungitoxicity of ONMS, NMS, and MS were determined in terms of their fungal growth inhibition capacity, which was determined by calculating the radial growth reduction in 5 day-old fungal colonies as compared to the control experiment [Fig. S5–S7, ESI†]. The bare MoS2(MS) sample, owing to its poor ROS generation capacity, failed to dominate over the fungal ROS scavenging machinery. Hence, no significant fungal inhibition was observed for the bare MS samples. However, doping nitrogen atoms into MoS2 is seen to result in accelerated fungitoxic effects under dark, as revealed by the digital photographs of NMS-treated fungal colonies, which gets further enhanced when treated under visible light irradiation. In the presence of visible light, NMS nanosheets (0.5 mg mL−1) shows 12.5% radial growth inhibition towards A. alternata, which is 2.12 times greater than that measured under dark experiments [Fig. 5(b)]. However, the fungitoxicity of the NMS nanostructures increased in a dose dependent manner. Under visible light illumination and for a concentration of 2 mg mL−1, NMS nanostructures showed nearly 55% mycelial growth retardation in A. alternata, which is 1.1 times higher than the growth retardation achieved with treatment under dark conditions. For F. oxysporum, at lower dosage (0.5 mg mL−1), NMS showed a 12.5% growth inhibition upon light irradiation, which is very similar to that for A. alternata. However, when treated with 2 mg mL−1 concentration, the fungitoxicity doubles on visible light exposure [Fig. 5(b)]. Higher fungitoxicity in the presence of visible light illumination supports the photo-induced acceleration of ROS generation, resulting in greater cell death. Being a super oxidizing agent, the introduction of oxygen in the MoS2 nanostructure, in addition to nitrogen, accelerated its fungitoxicity further, as expected in the case of ONMS.
Fig. 5(a) shows that for a 2 mg mL−1 concentration and upon visible light treatment, ONMS successfully suppressed 84.21% mycelial growth of A. alternata, which is 16.47% higher than the retardation obtained under dark experimental conditions. Likewise, under visible light, for F. oxysporum, ONMS nanoflowers achieved a fungal growth retardation of 72.54% with a dosage of 2 mg mL−1, which was 15.41% higher when treated under dark. Comparing the results obtained for NMS and the ONMS nanoflowers, it is evident that the introduction of oxygen into MoS2 increased their fungitoxicity ∼1.5 and ∼1.6 times for A. alternata and F. oxysporum, respectively [Fig. 5(a) and (b)].
The biochemical assessment of fungal cell viability after ONMS treatment were performed by TTC assay.63 Both the pathogens were treated with different doses of ONMS nanoflowers for 24 hours, followed by the addition of TTC reagent and further incubation for 12 hours. The dehydrogenase enzymes secreted by viable fungal cells reduced TTC into a red coloured compound, namely, formazan, which was dissolved in dimethyl sulfoxide (DMSO) and detected spectrophotometrically at 570 nm. Cell viability was correlated with formazan formation and is presented in Fig. S8 (ESI†). In a line similar to the radial growth inhibition assay, ONMS nanoflowers exhibited higher toxicity towards A. alternata than F. oxysporum, which are ∼86% and 75%, respectively.
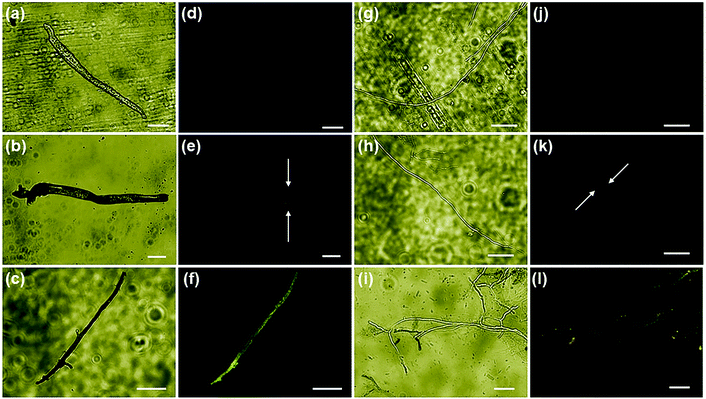
Further, the evaluation of the ONMS nanoflower-induced morphological deformities in the fungal cells with reference to the untreated biomass were carried out using an scanning electron microscope and is presented in [Fig. 5(c)–(h)]. For both the pathogens, the smooth surfaced untreated fungal mycelia with a distinct outline gets completely distorted on being treated with doped MoS2 nanoflowers. The release of intra-cellular materials, as evident from the SEM images of the ONMS-treated fungal biomass, is a clear indication of ROS-induced perforations within the cell wall, resulting in its higher permeability, which thereby leads to compulsive cell death. Detection of real-time ROS generation by ONMS nanosheets was performed using DCF dye (dichlorofluorescein) and their effect upon the fungal biomass has been captured under a fluorescence microscope and is presented in Fig. 6. In the case of untreated fungal mycelia, no fluorescence was detected, which indicated the absence of ROS in normal fungal mycelia. In contrast, when treated with ONMS nanoparticles, the emission of green fluorescence was detected upon blue excitation, indicating nanoparticle-induced ROS generation onto the mycelial surface, causing plausible cellular damage and deformities. Again, nanomaterial treatment under visible light illumination shows higher emission of green fluorescence, which supports photo-induced elevation in ROS generation by the nanosheets [Fig. 6].
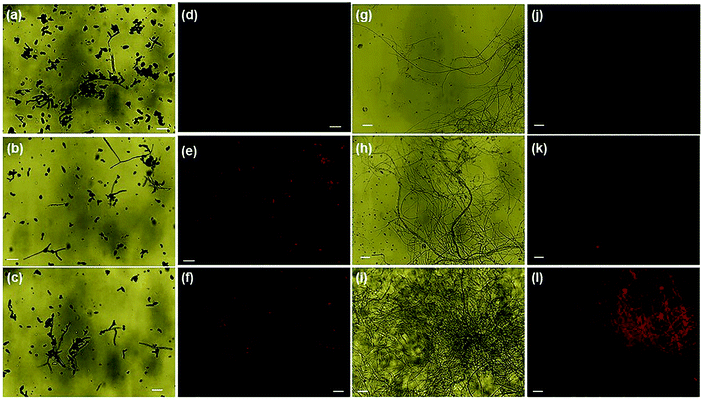
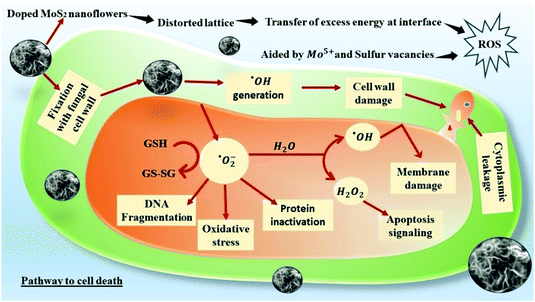
Nanoparticle-induced fungal cell death has also been confirmed by fluorescence microscopy using EtBr staining (ethidium bromide). The perforated cell wall of the nanoparticle-treated fungal biomass allows EtBr binding with DNA material, showing red fluorescence upon green excitation [Fig. 7]. The prominent emission of red fluorescence evident in ONMS and NMS-treated fungal mycelia under visible light irradiation can be attributed as a confirmation of photo-induced accelerated ROS generation [Fig. 6]. However, no red fluorescence was seen in the case of untreated fungal mycelia, which signifies the characteristic rigidity and integrity of the chitin layer, thus successfully eliminating EtBr entry at the intracellular matrix. ROS generation propensity of MoS2 and engineered N-doped MoS2 had been well-documented.30,37 The superoxide ˙O2− and hydroxyl ˙OH radicals generated under dark and visible light illumination possess strong oxidation potential. The production of excessive ROS at the cellular environment dominates over its ROS scavenging machinery, thus altering the redox balance at the intracellular level. The impairment in cellular redox-balance imposes the oxidative stress on the cell membrane and affects the membrane permeability severely, leading to compulsive cell death. The generation of superoxide molecules triggers a series of redox reactions inside the cell, synthesizing more toxic molecules that cause oxidation of DNA, lipid, proteins, enzymes, oxidation of water into hydroxyl radical (˙OH), and production of toxic H2O2 molecule. The de-activation of glutathione (GSH) and GSH-related enzymes is one of the most studied toxicological implications of superoxide molecules. GSH plays an important role in the cellular anti-oxidant system.64 The oxidation of the –SH group of GSH enzymes leads to stable disulphide (–S–S–) bond formation, resulting in functional inactivation of the enzyme. Consequently, the susceptibility of the fungal cells to the toxic oxidants multiplies several times, exposing it to higher risk of cellular damages. Other than that, the superoxide molecule potentially oxidizes important biomolecules such as DNA, RNA, protein, and lipids, and triggers cellular apoptosis and aging.37,65
Oxidation of water molecules generates ˙OH radicals that exhibit significant oxidation potential. It strongly reacts with the H atom of the N-acetyl-glucosamine monomer of the chitin layer and breaks the 1,4 and 1,6-glycosidic linkages, disturbing the characteristic rigidity and integrity of the fungal cell wall. In addition, ˙OH radicals also oxidizes intracellular bio-molecules of the microbial cell, thus altering the cellular biochemistry.63,66 A schematic representation of the ROS-mediated antifungal mechanism is given in Fig. 8. Superoxide molecules are reduced to H2O2 and their toxicity to the cell is multi-dimensional. It is actively involved in enhancing cellular apoptosis by DNA fragmentation and chromatin condensation. Higher accumulation of H2O2 at the cellular level also results in lipid peroxidation and protein oxidation, thus impairing the cellular stability.67
The antifungal effect of ONMS was by far better than that of NMS, both in light and in dark. As there is not much significant difference in terms of morphology, vacancy states, and porosity, the better antifungal performance of ONMS in comparison to NMS may be attributed to the elevated levels of ROS generation from the Mo5+ sites in ONMS. Interestingly, unlike our previous work,37 in which the S-vacancies played a pivotal role in the spontaneous generation of ROS by N-doped MoS2, ROS generation in the dark in the present study for ONMS was prompted upon by the different mechanism. Because of distortion due to oxygen and nitrogen co-doping, the ONMS lattice is under strain, as revealed from the Raman spectroscopy analysis. On being brought in contact with the aqueous environment (cellular water and constituent water part in the fungal growth media), the strain in doped MoS2 is relaxed and there is a spontaneous transfer of elastic energy stored in the lattice, which thereby triggers the generation of ROS in dark. ROS generation is further augmented under visible light illumination as a result of electron–hole pair generation, which react with dissolved oxygen to form the ROS species. MoS2 being a semiconductor, on being excited with energies greater than its band gap energy, the electrons on its valence band are excited to move to the conduction band, thus creating holes in the VB.30 The better antifungal performance of ONMS originates from the presence of Mo5+ sites in ONMS, as revealed from the EPR studies, which are the active hubs of ROS generation in this case.
The uniqueness and superiority of ONMS over most other nanomaterial-based fungicides lie in the fact that it can generate ROS in dark, which gets enhanced on being illuminated with visible light. Apart from its use in usual antifungal applications, this paves the way for its application in antifungal coatings in implantable medical devices. A comparative study of ONMS with other 2D-nanolayer-based antifungals is given in Table S1 of the ESI,† which shows that the effectiveness towards fungal inhibition of the present sample is in good competition with the presently available fungicides. Further, in order to address the issue of biocompatibility, it may be mentioned here that the constituent elements (Mo and S) of the nanomaterial used in this study are established micro-nutrients that promote plant growth and metabolism. Moreover, bare MoS2, as a compound, does not exhibit any toxicity, which is experimentally evident from our study [Fig. S7, ESI†]. The toxicity in other samples is purely ROS mediated and achieved by introducing N and O into the nanostructure. Further, the EC50 (concentration of ∼50% growth inhibition) value of ONMS for A. alternata and F. oxysporum are 1.0 and 1.5 mg mL−1, respectively. Although, the minute concentration of the nanomaterial is high enough to control the fungal pathogens, the plant's antioxidant system can easily scavenge the ROS generated by the applied nanomaterials. Moreover, there are numerous reports of the excellent biocompatibility of MoS2 and N-doped MoS2.68,69 However, to figure out the influence of ONMS on the plant body and to address the issue of biocompatibility, a small experiment was conducted with potato tubers, which ensured the biocompatibility of the present materials towards plant cell. The details of the experiment have been given in the ESI† (Section S1.3).
4. Conclusions
In summary, we have successfully incorporated oxygen and nitrogen atoms in MoS2 nanoflowers through an optimised and facile hydrothermal method and studied the antifungal effects of the synthesized samples on fungal pathogens, namely, A. alternata and F. oxysporum. The HRTEM, XRD, Raman, XPS, and EPR characterisation results confirmed the presence of O-doping in ONMS. The PAS studies revealed that the overall defect constitution in ONMS and NMS is almost similar with very little differences in the defect history of the two samples. Moreover, doping with oxygen and nitrogen atoms resulted in excellent antifungal activities in MoS2, which otherwise showed no antifungal growth inhibition whatsoever. The antifungal performance of ONMS was superior to that of NMS, both in dark and under visible light illumination. The superiority of ONMS over NMS was achieved through the careful design of the MoS2 nanoflowers through oxygen doping to cultivate the Mo5+ states within the lattice. These Mo5+ defect states in ONMS acted as the primary sites for ROS generation, thereby leading to an enhanced performance towards the inhibition of A. alternata and F. oxysporum. The present study will thus open up the avenue for the design of newer antifungals with lesser chances of antimicrobial resistance through suitable elemental doping in order to cope with the growing threats to global biodiversity due to unprecedented global warming.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the CSIR-India [03(1391)/16/EMR-II], UGC-SAP, India [530/20/DRS-II/2015 (SAP-I)] and DST-FIST, India [SR/FST/PSI-219/2016].References
- C. Kinealy, This great calamity: the great Irish Famine: the Irish Famine, Gill & Macmillan Ltd, 1994, pp. 1845–1852 Search PubMed.
- J. R. Perfect, The antifungal pipeline: a reality check, Nat. Rev. Drug Discovery, 2017, 16(9), 603 CrossRef CAS PubMed.
- C.-Y. Low and C. Rotstein, Emerging fungal infections in immunocompromised patients, F1000Med. Rep., 2011, 3 Search PubMed.
- C. A. Damalas and I. G. Eleftherohorinos, Pesticide exposure, safety issues, and risk assessment indicators, Int. J. Environ. Res. Public Health, 2011, 8(5), 1402–1419 CrossRef CAS PubMed.
- K. Niemirowicz, B. Durnaś, E. Piktel and R. Bucki, Development of antifungal therapies using nanomaterials, Nanomedicine, 2017, 12(15), 1891–1905 CrossRef CAS PubMed.
- A. Panáček, M. Kolář, R. Večeřová, R. Prucek, J. Soukupova, V. Kryštof, P. Hamal, R. Zbořil and L. Kvítek, Antifungal activity of silver nanoparticles against Candida spp, Biomaterials, 2009, 30(31), 6333–6340 CrossRef PubMed.
- A. Karimiyan, H. Najafzadeh, M. Ghorbanpour and S. H. Hekmati-Moghaddam, Antifungal effect of magnesium oxide, zinc oxide, silicon oxide and copper oxide nanoparticles against Candida albicans. Zahedan, J. Res. Med. Sci., 2015, 17(10), 19–23 Search PubMed.
- L. He, Y. Liu, A. Mustapha and M. Lin, Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum, Microbiol. Res., 2011, 166(3), 207–215 CrossRef CAS PubMed.
- S. Zhu, F. Luo, B. Zhu and G.-X. Wang, Toxicological effects of graphene oxide on Saccharomyces cerevisiae, Toxicol. Res., 2017, 6(4), 535–543 CrossRef CAS PubMed.
- M. Sawangphruk, P. Srimuk, P. Chiochan, T. Sangsri and P. Siwayaprahm, Synthesis and antifungal activity of reduced graphene oxide nanosheets, Carbon, 2012, 50(14), 5156–5161 CrossRef CAS.
- C. Li, X. Wang, F. Chen, C. Zhang, X. Zhi, K. Wang and D. Cui, The antifungal activity of graphene oxide–silver nanocomposites, Biomaterials, 2013, 34(15), 3882–3890 CrossRef CAS PubMed.
- N. Gómez-Ortíz, S. De la Rosa-García, W. González-Gómez, M. Soria-Castro, P. Quintana, G. Oskam and B. Ortega-Morales, Antifungal coatings based on Ca(OH)2 mixed with ZnO/TiO2 nanomaterials for protection of limestone monuments, ACS Appl. Mater. Interfaces, 2013, 5(5), 1556–1565 CrossRef PubMed.
- N. Meng, N.-L. Zhou, S.-Q. Zhang and J. Shen, Synthesis and antifungal activities of polymer/montmorillonite–terbinafine hydrochloride nanocomposite films, Appl. Clay Sci., 2009, 46(2), 136–140 CrossRef CAS.
- R. Prucek, J. Tuček, M. Kilianová, A. Panáček, L. Kvítek, J. Filip, M. Kolář, K. Tománková and R. Zbořil, The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles, Biomaterials, 2011, 32(21), 4704–4713 CrossRef CAS PubMed.
- V. Rodríguez-González, R. B. Domínguez-Espíndola, S. Casas-Flores, O. A. Patrón-Soberano, R. Camposeco-Solis and S.-W. Lee, Antifungal nanocomposites inspired by titanate nanotubes for complete inactivation of Botrytis cinerea isolated from tomato infection, ACS Appl. Mater. Interfaces, 2016, 8(46), 31625–31637 CrossRef PubMed.
- K.-K. Liu, W. Zhang, Y.-H. Lee, Y.-C. Lin, M.-T. Chang, C.-Y. Su, C.-S. Chang, H. Li, Y. Shi and H. Zhang, Growth of large-area and highly crystalline MoS2 thin layers on insulating substrates, Nano Lett., 2012, 12(3), 1538–1544 CrossRef CAS PubMed.
- Z. Yin, H. Li, H. Li, L. Jiang, Y. Shi, Y. Sun, G. Lu, Q. Zhang, X. Chen and H. Zhang, Single-layer MoS2 phototransistors, ACS Nano, 2012, 6(1), 74–80 CrossRef CAS PubMed.
- Q. Chen, Y. Ouyang, S. Yuan, R. Li and J. Wang, Uniformly wetting deposition of Co atoms on MoS2 monolayer: a promising two-dimensional robust half-metallic ferromagnet, ACS Appl. Mater. Interfaces, 2014, 6(19), 16835–16840 CrossRef CAS PubMed.
- T. Pham, G. Li, E. Bekyarova, M. E. Itkis and A. Mulchandani, MoS2-based optoelectronic gas sensor with sub-parts-per-billion limit of NO2 gas detection, ACS Nano, 2019, 13(3), 3196–3205 CrossRef CAS PubMed.
- L. Zhang, X. Ji, X. Ren, Y. Ma, X. Shi, Z. Tian, A. M. Asiri, L. Chen, B. Tang and X. Sun, Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: theoretical and experimental studies, Adv. Mater., 2018, 30(28), 1800191 CrossRef PubMed.
- D. Kufer and G. Konstantatos, Highly sensitive, encapsulated MoS2 photodetector with gate controllable gain and speed, Nano Lett., 2015, 15(11), 7307–7313 CrossRef CAS PubMed.
- Z. Hu, L. Wang, K. Zhang, J. Wang, F. Cheng, Z. Tao and J. Chen, MoS2 nanoflowers with expanded interlayers as high-performance anodes for sodium-ion batteries, Angew. Chem., Int. Ed., 2014, 53(47), 12794–12798 CrossRef CAS PubMed.
- X. Zhu, X. Ji, N. Kong, Y. Chen, M. Mahmoudi, X. Xu, L. Ding, W. Tao, T. Cai and Y. Li, Intracellular mechanistic understanding of 2D MoS2 nanosheets for anti-exocytosis-enhanced synergistic cancer therapy, ACS Nano, 2018, 12(3), 2922–2938 CrossRef CAS PubMed.
- X. Li and H. Zhu, Two-dimensional MoS2: properties, preparation, and applications, J. Materiomics, 2015, 1(1), 33–44 CrossRef.
- A. Splendiani, L. Sun, Y. Zhang, T. Li, J. Kim, C.-Y. Chim, G. Galli and F. Wang, Emerging photoluminescence in monolayer MoS2, Nano Lett., 2010, 10(4), 1271–1275 CrossRef CAS PubMed.
- C. Zhu, Z. Zeng, H. Li, F. Li, C. Fan and H. Zhang, Single-layer MoS2-based nanoprobes for homogeneous detection of biomolecules, J. Am. Chem. Soc., 2013, 135(16), 5998–6001 CrossRef CAS PubMed.
- S. S. Chou, B. Kaehr, J. Kim, B. M. Foley, M. De, P. E. Hopkins, J. Huang, C. J. Brinker and V. P. Dravid, Chemically exfoliated MoS2 as near-infrared photothermal agents, Angew. Chem., Int. Ed., 2013, 52(15), 4160–4164 CrossRef CAS PubMed.
- V. Yadav, S. Roy, P. Singh, Z. Khan and A. Jaiswal, 2D MoS2-based nanomaterials for therapeutic, bioimaging, and biosensing applications, Small, 2019, 15(1), 1803706 CrossRef PubMed.
- X. Wang, T. Li, H. Ma, D. Zhai, C. Jiang, J. Chang, J. Wang and C. Wu, A 3D-printed scaffold with MoS2 nanosheets for tumor therapy and tissue regeneration, NPG Asia Mater., 2017, 9(4), e376 CrossRef CAS.
- C. Liu, D. Kong, P.-C. Hsu, H. Yuan, H.-W. Lee, Y. Liu, H. Wang, S. Wang, K. Yan and D. Lin, Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light, Nat. Nanotechnol., 2016, 11(12), 1098–1104 CrossRef CAS PubMed.
- Q. Xu, P. Zhu, J. Zhang, Y. Liu, L. Cai, H. Jiang, M. Ji and J. Chen, Electrochemical formation of distinct nanostructured MoS2 with altered antibacterial activity, Mater. Lett., 2020, 127809 CrossRef CAS.
- S. Karunakaran, S. Pandit, B. Basu and M. De, Simultaneous exfoliation and functionalization of 2H-MoS2 by thiolated surfactants: applications in enhanced antibacterial activity, J. Am. Chem. Soc., 2018, 140(39), 12634–12644 CrossRef CAS PubMed.
- S. Begum, A. Pramanik, K. Gates, Y. Gao and P. C. Ray, Antimicrobial Peptide-Conjugated MoS2-Based Nanoplatform for Multimodal Synergistic Inactivation of Superbugs, ACS Appl. Bio Mater., 2018, 2(2), 769–776 CrossRef.
- X. Zhang, W. Zhang, L. Liu, M. Yang, L. Huang, K. Chen, R. Wang, B. Yang, D. Zhang and J. Wang, Antibiotic-loaded MoS2 nanosheets to combat bacterial resistance via biofilm inhibition, Nanotechnology, 2017, 28(22), 225101 CrossRef PubMed.
- T. I. Kim, B. Kwon, J. Yoon, I.-J. Park, G. S. Bang, Y. Park, Y.-S. Seo and S.-Y. Choi, Antibacterial activities of graphene oxide–molybdenum disulfide nanocomposite films, ACS Appl. Mater. Interfaces, 2017, 9(9), 7908–7917 CrossRef CAS PubMed.
- W. Zhang, Z. Mou, Y. Wang, Y. Chen, E. Yang, F. Guo, D. Sun and W. Wang, Molybdenum disulfide nanosheets loaded with chitosan and silver nanoparticles effective antifungal activities: in vitro and in vivo, Mater. Sci. Eng. Carbon, 2019, 97, 486–497 CrossRef CAS PubMed.
- P. Basu, J. Chakraborty, N. Ganguli, K. Mukherjee, K. Acharya, B. Satpati, S. Khamrui, S. Mandal, D. Banerjee and D. Goswami, Defect-Engineered MoS2 Nanostructures for Reactive Oxygen Species Generation in the Dark: Antipollutant and Antifungal Performances, ACS Appl. Mater. Interfaces, 2019, 11(51), 48179–48191 CrossRef CAS PubMed.
- A. Ramasubramaniam and D. Naveh, Mn-doped monolayer MoS2: an atomically thin dilute magnetic semiconductor, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87(19), 195201 CrossRef.
- J. Zhou, G. Fang, A. Pan and S. Liang, Oxygen-incorporated MoS2 nanosheets with expanded interlayers for hydrogen evolution reaction and pseudocapacitor applications, ACS Appl. Mater. Interfaces, 2016, 8(49), 33681–33689 CrossRef CAS PubMed.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution, J. Am. Chem. Soc., 2013, 135(47), 17881–17888 CrossRef CAS PubMed.
- T. Sun, Z. Li, X. Liu, L. Ma, J. Wang and S. Yang, Oxygen-incorporated MoS2 microspheres with tunable interiors as novel electrode materials for supercapacitors, J. Power Sources, 2017, 352, 135–142 CrossRef CAS.
- N. M. Brown, N. Cui and A. McKinley, An XPS study of the surface modification of natural MoS2 following treatment in an RF-oxygen plasma, Appl. Surf. Sci., 1998, 134(1–4), 11–21 CrossRef CAS.
- H. Zhu, X. Qin, L. Cheng, A. Azcatl, J. Kim and R. M. Wallace, Remote plasma oxidation and atomic layer etching of MoS2, ACS Appl. Mater. Interfaces, 2016, 8(29), 19119–19126 CrossRef CAS PubMed.
- J. Martin, J. Cross and L. Pope, MoS2 Interactions with 1.5 eV Atomic Oxygen, MRS Online Proc. Libr., 1988, 140 Search PubMed.
- W. Su, N. Kumar, S. J. Spencer, N. Dai and D. Roy, Transforming bilayer MoS2 into single-layer with strong photoluminescence using UV-ozone oxidation, Nano Res., 2015, 8(12), 3878–3886 CrossRef CAS.
- J. Wu, H. Li, Z. Yin, H. Li, J. Liu, X. Cao, Q. Zhang and H. Zhang, Layer thinning and etching of mechanically exfoliated MoS2 nanosheets by thermal annealing in air, Small, 2013, 9(19), 3314–3319 CAS.
- C. Lee, H. Yan, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, Anomalous lattice vibrations of single-and few-layer MoS2, ACS Nano, 2010, 4(5), 2695–2700 CrossRef CAS PubMed.
- A. Azcatl, X. Qin, A. Prakash, C. Zhang, L. Cheng, Q. Wang, N. Lu, M. J. Kim, J. Kim and K. Cho, Covalent nitrogen doping and compressive strain in MoS2 by remote N2 plasma exposure, Nano Lett., 2016, 16(9), 5437–5443 CrossRef CAS PubMed.
- M. Dieterle, G. Weinberg and G. Mestl, Raman spectroscopy of molybdenum oxides Part I. Structural characterization of oxygen defects in MoO3−x by DR UV/VIS, Raman spectroscopy and X-ray diffraction, Phys. Chem. Chem. Phys., 2002, 4(5), 812–821 RSC.
- J. Xiong, Y. Liu, D. Wang, S. Liang, W. Wu and L. Wu, An efficient cocatalyst of defect-decorated MoS2 ultrathin nanoplates for the promotion of photocatalytic hydrogen evolution over CdS nanocrystal, J. Mater. Chem. A, 2015, 3(24), 12631–12635 RSC.
- M. Nagai, A. Irisawa and S. Omi, XPS Study of the Deactivation and Sulfiding of Nitrided Molybdena–Alumina Catalyst during the Hydrodesulfurization of Dibenzothiophene. The, J. Phys. Chem. B, 1998, 102(39), 7619–7626 CrossRef CAS.
- B. J. Tan, K. J. Klabunde and P. M. Sherwood, XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt–manganese particles on alumina and silica, J. Am. Chem. Soc., 1991, 113(3), 855–861 CrossRef CAS.
- S. Knipe, J. Mycroft, A. Pratt, H. Nesbitt and G. Bancroff, X-ray photoelectron spectroscopic study of water adsorption on iron sulphide minerals, Geochim. Cosmochim. Acta, 1995, 59(6), 1079–1090 CrossRef CAS.
- C. Zhang, J. Sunarso and S. Liu, Designing CO2-resistant oxygen-selective mixed ionic–electronic conducting membranes: guidelines, recent advances, and forward directions, Chem. Soc. Rev., 2017, 46(10), 2941–3005 RSC.
- S. J. Hilsenbeck, V. G. Young Jr and R. E. McCarley, Synthesis, structure, and characterization of N-ligated Mo6S8L6 cluster complexes. Molecular precursors to chevrel phases, Inorg. Chem., 1994, 33(9), 1822–1832 CrossRef CAS.
- L. Benoist, D. Gonbeau, G. Pfister-Guillouzo, E. Schmidt, G. Meunier and A. Levasseur, X-ray photoelectron spectroscopy characterization of amorphous molybdenum oxysulfide thin films, Thin Solid Films, 1995, 258(1–2), 110–114 CrossRef CAS.
- T. A. Patterson, J. C. Carver, D. E. Leyden and D. M. Hercules, A surface study of cobalt–molybdena–alumina catalysts using X-ray photoelectron spectroscopy, J. Phys. Chem., 1976, 80(15), 1700–1708 CrossRef CAS.
- S. McDonnell, A. Azcatl, R. Addou, C. Gong, C. Battaglia, S. Chuang, K. Cho, A. Javey and R. M. Wallace, Hole contacts on transition metal dichalcogenides: interface chemistry and band alignments, ACS Nano, 2014, 8(6), 6265–6272 CrossRef CAS PubMed.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis, Nat. Mater., 2012, 11(11), 963–969 CrossRef CAS PubMed.
- J. R. González, R. Alcántara, J. L. Tirado, A. J. Fielding and R. A. Dryfe, Electrochemical interaction of few-layer molybdenum disulfide composites vs. sodium: new insights on the reaction mechanism, Chem. Mater., 2017, 29(14), 5886–5895 CrossRef.
- C. Louis and M. Che, EPR investigation of the coordination sphere of molybdenum (5+) ions on thermally reduced silica-supported molybdenum catalysts prepared by the grafting method, J. Phys. Chem., 1987, 91(11), 2875–2883 CrossRef CAS.
- R. Nieminen and J. Laakkonen, Positron trapping rate into vacancy clusters, Appl. Phys., 1979, 20(2), 181–184 CAS.
- S. S. Boxi, K. Mukherjee and S. Paria, Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens, Nanotechnology, 2016, 27(8), 085103 CrossRef PubMed.
- X. Yang, J. Li, T. Liang, C. Ma, Y. Zhang, H. Chen, N. Hanagata, H. Su and M. Xu, Antibacterial activity of two-dimensional MoS2 sheets, Nanoscale, 2014, 6(17), 10126–10133 RSC.
- N. R. Panyala, E. M. Peña-Méndez and J. Havel, Silver or silver nanoparticles: a hazardous threat to the environment and human health?, J. Appl. Biomedicine, 2008, 6, 3 Search PubMed.
- K. Mukherjee, K. Acharya, A. Biswas and N. R. Jana, TiO2 Nanoparticles Co-doped with Nitrogen and Fluorine as Visible-Light-Activated Antifungal Agents, ACS Appl. Nano Mater., 2020, 3(2), 2016–2025 CrossRef CAS.
- M. Kawai-Yamada, Y. Ohori and H. Uchimiya, Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death, The Plant Cell, 2004, 16(1), 21–32 CrossRef CAS PubMed.
- J. H. Appel, D. O. Li, J. D. Podlevsky, A. Debnath, A. A. Green, Q. H. Wang and J. Chae, Low cytotoxicity and genotoxicity of two-dimensional MoS2 and WS2, ACS Biomater. Sci. Eng., 2016, 2(3), 361–367 CrossRef CAS.
- F.-Y. Wu, Y.-S. Cheng, D.-M. Wang, M.-L. Li, W.-S. Lu, X.-Y. Xu, X.-H. Zhou and X.-W. Wei, Nitrogen-doped MoS2 quantum dots: Facile synthesis and application for the assay of hematin in human blood, Mater. Sci. Eng. Carbon, 2020, 110898 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of positron annihilation spectroscopy experiments, coincidence Doppler broadening spectroscopy experiments, fungitoxicity assays including growth inhibition test, TTC assay, fluorescence microscopy studies, comparison chart with other antifungal materials, and other useful results. See DOI: 10.1039/d0ma00343c |
| This journal is © The Royal Society of Chemistry 2020 |