Self-assembly of nano-ellipsoids into ordered structures via vertical deposition†
Venkateshwar Rao Dugyala and
Madivala G. Basavaraj*
Polymer Engineering and Colloid Science Lab (PECS Lab), Department of Chemical Engineering, Indian Institute of Technology Madras, India. E-mail: basa@iitm.ac.in
First published on 6th July 2015
Abstract
The self-assembly of anisotropic particles into two or three dimensional ordered arrays has diverse applications in several areas of science and engineering. In this article, we report the formation of a multi-layer film of nano-sized ellipsoids with long-range orientation and positional order through an evaporation driven vertical deposition technique. Using nano-sized ellipsoids of tunable surface charge, we show that electrostatic interactions can be exploited to control the arrangement of ellipsoids in the film. The pH of the aqueous suspensions used for vertical deposition is adjusted such that the surface charge and hence the electro-static interactions can be controlled. We show that the interplay between various Derjaguin and Landau, Verwey and Overbeek (DLVO) interactions can be effectively controlled to fabricate films with a random arrangement of particles as well as to create particulate films with three-dimensional ordering of ellipsoids. When the particles are weakly charged, the nano-ellipsoids in the film are randomly oriented giving an amorphous particulate film. However, when the particles are highly charged, the nano-ellipsoids in the film are oriented with major axis parallel to each other and ordered in three dimensions. These particulate film are observed to have parallel cracks across the entire width of the film. The major axis of the particle is found to be parallel to the crack direction and therefore, the direction of crack formation can be exploited to indicate the nature of particle ordering.
Introduction
Colloidal scale particles can be self-assembled in two or three dimensions into a wide range of supra-structures through several bottom-up self-assembly routes. Such supra-structures of nano and micron sized particles have a wide range of applications in different fields such as photonics, catalysis, diagnostics and sensors.1–4 In recent years, there has been an enormous interest, in particular, in the fabrication of novel structures via the directed self-assembly of shape anisotropic particles.5–7 Self-assembled structures of anisotropic particles with long-range order have specific applications for example in hypersonic phonon propagation as well as in photonics.6,8 In literature there exist various strategies to create ordered structures of anisotropic particles. Using a suspension of ellipsoids that respond to applied magnetic field or electric field, it is possible to direct the assembly of ellipsoids into 2D (linear) chains and 3D ordered arrays with positional and orientation order.9–13 A combination of field and flow assisted assembly has been used to create particulate films of oriented titanium dioxide ellipsoids that exhibit anisotropic mechanical properties.11 More recently, a method for the reversible formation of self-assembly of colloidal ellipsoids into tubular configuration that resemble several biological structures have been created with the help of external electric field.14 Shape induced capillary interactions have been exploited to obtain a percolating two dimensional self-assembly of end-to-end connected micron sized ellipsoids and nanorods.15,16 In this article, we report a simple one step method for the fabrication of particle films containing oriented colloidal ellipsoids using controlled evaporation driven self-assembly.Vertical deposition is a simple and effective evaporation driven self-assembly technique for the three-dimensional organization of particles on a substrate. In the vertical deposition, a vial containing a colloidal dispersion and a substrate is subjected to controlled evaporation. Due to the immersion of the substrate in the colloidal dispersion, a three phase contact line is formed at the substrate–solvent–air interface. As the solvent evaporates, the capillary flow generated to replenish the evaporated solvent carries the colloidal particles towards the contact line thereby leaving a film deposit at the three-phase contact line.17 It has been shown that for spherical particles, the convex flow through the particles leads to the formation of colloidal crystals with FCC arrangement.18–20 It is possible to achieve control over the nature of particle arrangement in the particle film – for example – by regulating the meniscus movement (evaporation condition) and sedimentation rate.21–25 Due to the long-range ordering of particles obtained via vertical deposition, the particulate films fabricated through these routes exhibit cracks.26 However, it is possible to eliminate cracks by adding a suitable precursor to the suspension.
Most of the literature on the use of vertical deposition deal with the three dimensional assembly of spherical particles. Recently, vertical deposition under controlled temperature gradients (to avoid sedimentation) has been employed to create a three dimensional ordered assembly of cubic colloids with square and hexagonal lattice arrangements.27 Similarly, magnetic ellipsoids through a combination of vertical deposition and external magnetic field show a tendency to form long range 3D photonic crystals.8 The type of particle film – continuous or discontinuous (striped) depends on the concentration of particles in the suspension and the use of other additives such as surfactants. In a suspension of single wall carbon nanotube (SWCN) containing SDS, the film patterns changes from stripe to continuous films with increase in concentration of SWCN.28 At low particles concentration, the pinning and de-pinning of the three phase contact line gives the stripe patterns, as the particles concentration increase the film become continuous. The motivation for the use of vertical deposition to assemble colloidal ellipsoids is two-fold. As the crystal structure are strongly dependent on the particle shape, we would like to exploit vertical deposition to create ordered assembly of ellipsoids with different arrangements. Moreover, the role of colloidal interactions on the three dimensional self-assembly of anisotropic particles via vertical deposition has not been investigated to date. Since it is known that the interactions between the particles play an important factor in their self-assembly, we would like to exploit the DLVO interactions to control the arrangement of particles during vertical deposition. In the present work we report the 3D self-assembly of hematite nano-ellipsoids of tunable surface charge through the vertical deposition technique without any external filed. A 3D self-assembly of oriented particles is observed when the particle–particle interactions are highly repulsive. A disordered structure is obtained when particles are weakly charged. We also show that the particle films exhibit cracks when the particles are highly ordered and there are no cracks in the amorphous films obtained respectively when the particles are highly or weakly charged.
Experimental methods
Synthesis and characterization of hematite ellipsoids
Forced hydrolysis method was used to synthesis the hematite ellipsoid particles.29,30 The aspect ratio of the particles depends on the molar ratio of NaH2PO4 to Fe3+. In a 100 ml MilliQ water (18.2 MΩ cm), 3.54 g of Fe(ClO4)3 (Sigma Aldrich, India) and 0.6 g of urea (Merk, India) was added. Different amount of NaH2PO4 (Merk, India) was added to the Fe3+ solution to control particle aspect ratio. The reaction mixture was kept in a preheated oven at 100 °C for 24 h. After 24 h the reaction mixture is centrifuged at 4000g for 30 min and washed with MilliQ water multiple times. After final wash, particles were re-dispersed in MilliQ water. Scanning electron microscopy (Hitachi S-4800, Japan) was used to characterize the particles size and aspect ratio. The particles aspect ratio and sizes are shown in Table 1.| S no | Length (nm) | Diameter (nm) | Aspect ratio |
|---|---|---|---|
| 1 | 109 ± 10 | 55 ± 4 | 1.9 ± 0.17 |
| 2 | 247 ± 22 | 50 ± 7 | 4.7 ± 0.7 |
One of the advantage of using the hematite particles is that their surface charge can be varied by changing the suspension pH. Therefore, by adjusting the suspension pH with the addition of either HNO3 or NaOH, the surface charge is measured with electrophoretic dynamic light scattering technique (Nano partilca SZ-100, Horiba, Japan). In all the sample 0.0001 M salt concentration was maintained. The measured particle zeta potentials as a function of pH is shown in ESI Fig. S1.†
Vertical deposition
The concentration of particles in the suspensions used for vertical deposition experiments is 0.0248% v/v (aspect ratio ∼5) and 0.052% v/v (aspect ratio ∼2). All vertical deposition experiments are performed by taking 2 ml hematite suspension in a vial. The glass slide (of typical dimensions – 10 mm × 5 mm × 2 mm) used as a substrates are cleaned with pirhana solution (70% H2SO4 and 30% H2O2) and subsequently washed multiple times with MilliQ water (18.2 MΩ cm). The glass slide is placed vertically at an angle of approximately 90° such that a part of the substrate is immersed in the hematite suspension contained in the vial. The immersion area is ∼10 mm × 5 mm. All the experiments are conducted in a closed chamber maintained at a fixed temperature 28 ± 2 °C and relative humidity 65 ± 5%. After the complete evaporation of the solvent, the glass slide with solid deposit is used for the characterization of particulate film. Bright field optical microscopy (Leica, France) is used to observe the film characteristics such as the film length and surface morphology such as cracks. In ordered to visualize the arrangement of particles in the films at the length scale of particle size, a high resolution scanning electron microscope (Hitachi, Japan) is used. The samples are sputter coated with gold for 30 s prior to SEM imaging.Results and discussions
Effect of suspension pH on the micro and macrostructure
To understand the role of DLVO interactions on the surface morphology and self-assembly of nano-ellipsoids in the films obtained via vertical deposition, hematite suspensions at two different pH conditions are considered. The choice pH conditions is based on the measurement of electrokinetic behaviour of nano-ellipsoids as a function of pH of the suspension (results of which can be found in Fig. S1 in the ESI†). Suspensions at pH = 2 and pH = 6.5 are selected. The suspensions at a pH of 2 contain nano-ellipsoids that are highly charged (ζ = +42 mV) and at a pH of 6.5 the particles are weakly charged (ζ = +12 mV). It must be noted that the glass substrate under acidic conditions (pH = 2) is weakly charged. At pH = 6.5, the charge on the substrate is opposite to that of particle and much higher than corresponding charge at pH = 2.As soon as the glass substrate is immersed in the suspension of nano-ellipsoids, a three phase contact line is created at the suspension–air–substrate interface (see Fig. 1A). The suspension is then subjected to evaporation under controlled conditions (T = 28 ± 2 °C, RH = 65 ± 5%). The three phase contact line is in general pinned to the substrate surface due to surface roughness. To keep the contact line pinned during the evaporation process, there is always a net flow of solvent towards the contact line from the bulk. This net capillary flows is generated due to the evaporation of solvent from the meniscus. This capillary flow carries the particles towards the contact line. The particles that get deposited at the contact line form a particulate film as the solvent evaporates. An example of typical dried film obtained from the evaporation of suspensions containing hematite nano-ellipsoids is shown in Fig. 1B. The reddish appearance of the film is due to the color of hematite particles. There is usually a variation in the thickness of the particulate films generated during vertical deposition due to – (1) decrease in height of the suspension in the vial during evaporation and subsequent de-pinning of the contact line (2) increase in concentration of the particles in the suspension with time.
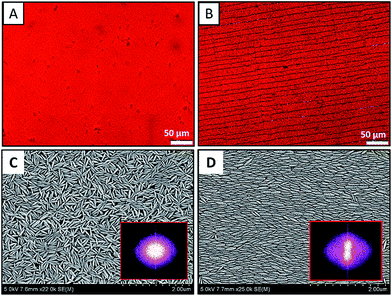
The dried films after complete evaporation of the solvent are analyzed for macroscopic and microscopic features. The images of the dried films containing ellipsoids of aspect ratio 4 are shown in Fig. 2. Fig. 2A and B respectively shown the bright filed microscopy images of dried films at pH = 6.5 and pH = 2. While the films appeared homogeneous visually, when imaged with microscopy at higher magnification, it is evident that at pH of 6.5 – a continuous film is formed and at pH of 2 – a continuous film with linear cracks is observed. As shown in Fig. 2B, the cracks are parallel to each other and run across the entire width of the film. The cracks are found to be parallel to the contact line.
To observe the local arrangement of particles and to see if there is any long-range order, SEM analysis is carried out. Fig. 2C and D respectively are the SEM images of dried films at pH = 6.5 and pH = 2. In Fig. 2C, the particles are randomly oriented. In Fig. 2D, the particles are oriented and aligned in one direction. The Fast Fourier Transform (FFT) images of the SEM images are shown in corresponding image as inset. The FFTs show further evidence for the random and aligned nature of ellipsoids as confirmed by the isotropic and anisotropic nature of the FFT patterns. The assembly of hematite nano-ellipsoids at pH 2 at the surface and interior of the film deposit is shown in Fig. 3. Fig. 3A and B are the top view of the film, where particles are arranged in an aligned closed packing manner. It is remarkable that the alignment of ellipsoids is three dimensional – that is – ellipsoids in each and every layer of the 3D film are oriented in the same direction and are closed packed as shown in the side view of the film in Fig. 3C and D. Therefore, the alignment of hematite ellipsoids is across the surface and thickness of the entire film. To further confirm the three-dimensional ordering of particles, the particulate film is sandwiched between two scotch tapes and the film is peeled off to visualize particle arrangement in the interior of the film. The SEM images (Fig. S2 in the ESI†) clearly demonstrate the ordering of nano-ellipsoids in 3 dimensions. The major axis of the nano-ellipsoids is observed to be parallel to the contact line. When the particles approach the contact line from the bulk, the torque on one end of the particle due to solvent flow turns the particle parallel to the contact line and the other particles follow the same arrangement.31 Similar observation have been reported in the deposits formed from sessile drops of anisotropic rod like particles and evaporation of suspensions of nano-rods under electric field.31,32
 | ||
| Fig. 3 SEM images showing the three dimensional ordering of hematite nano-ellipsoids of aspect ratio 4 observed upon the vertical deposition of suspensions at pH = 2. The particle arrangement at the surface of the film is shown in Fig. 3A and B. The SEM images or the side view of the film shown in Fig. 3C and D confirms the 3D ordering of particles across the surface and thickness of the film. | ||
Role of DLVO interactions on the particle arrangement
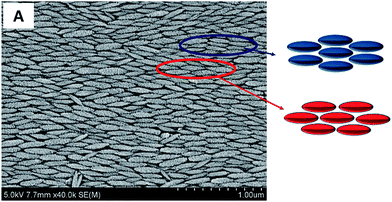
From the zeta-potential measurements (Fig. S1 in ESI†), it is clear that the particles and the substrate surface charge under the pH conditions used in the vertical deposition experiments are different. At pH = 2, the particles are highly charged with a zeta potential value of +42 mV and the substrate is weakly charged with a zeta potential value of ∼−2 mV. At pH = 6.5, the particles are comparatively weakly charged with a zeta potential of +12 mV and the substrate has a zeta potential of −30 mV. Since the vertical deposition process involves both the charged particles and the charged substrate, the particle–particle (PP) and particle–substrate (PS) interactions are important. Since the particles are anisotropic, the PP and PS interactions depend on the orientation of the particles. By considering parallel orientation of particle with the particle and particle with the substrate, we calculate the PP and PS interactions at different pH conditions using the Derjaguin's approximation (see ESI†).33 At pH = 2 the particles are highly charged, therefore, the net PP interaction at close separation is repulsive. However, at this pH, the glass slide is weakly negative charged, and therefore, the PS interactions are weakly attractive. As a result, the net DLVO interaction is repulsive, and therefore the individual nano-ellipsoids that reach the contact line can explore different packing configuration that are possible. The alignment is due to torque31 on the particles due to capillary flow and the repulsive particle–particle interactions. At pH = 6.5 the particles are weakly positive charged (ζ = +12 mV) and the substrate is negatively charged (comparatively carries higher surface charge of ζ = −30 mV). It must also be noted that the attractive van der walls interactions due to higher Hamaker constant of hematite particles will also contribute to the net interaction potential. So the overall DLVO interaction between the PP and PS is attractive. Moreover, when the particles reach the contact line either as aggregates or as individual particles, due to close particle–particle and particle–substrate separations, the net overall interactions are attractive. Therefore, the particles/aggregates deposit at the contact line in a random orientation as no rearrangements are possible (see Fig. 2C). Therefore, the particles accumulate at the contact line in a disorder manner as the evaporation continues leading to the formation of amorphous particle films without any particular particle arrangement.A close examination of the films containing ordered ellipsoids reveal that the particles are arranged in different lattice structures shown in Fig. 4. In Fig. 4 we have highlighted two types of hexagonal closed packing of ellipsoids that are locally observed. Since these configurations are repeatedly observed, these constitute the energetically favourable particle–particle arrangement. It is known that in 3D self-assembly structures the overall interaction is the sum of the all inter-particles interactions in a lattice. The overall interaction for a closed packed structure is given by34
 | (1) |
Effect of particle aspect ratio
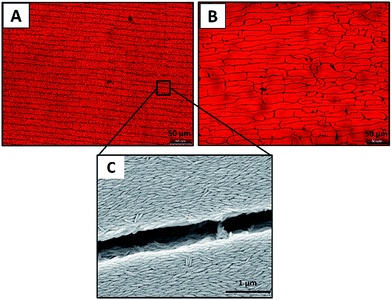
Vertical deposition experiments are conducted by evaporating aqueous suspensions of hematite nano-ellipsoids maintained at pH = 2 under identical conditions (2 ml suspension, T = 28 ± 2 °C, RH = 65 ± 5%), however, particles of two different aspect ratio are tested. The SEM images of the dried films containing ellipsoids of aspect ratio ∼2 and 4 are shown in Fig. 5. As shown in Fig. 5A, at low aspect ratio, the particles form several smaller domains where the particles show local ordering. In each domain the particles are arranged in one direction but the size and orientation of domains are observed to be different (as highlighted in Fig. 5A). Due to the presence of several domains each of different orientation, the FFT of the image in Fig. 5A is found to be isotropic that is there is no long range ordering. At high aspect ratio, the ordering of ellipsoids on an average is unidirectional and the domain area over which the particle ordering is observed in much larger in size (Fig. 5B).As shown in Fig. 6, this aspect ratio dependent local ordering of ellipsoids indeed has an effect on the macroscopic appearance of the films. While cracks are observed in both films, for aspect ratio 4 particles (Fig. 6A), the cracks are in one direction, they are parallel to the contact line. The cracks are observed to be parallel to each other as well and they run across the entire width of the film. For aspect ratio 2 particles, the crack patterns appear in both directions (parallel and perpendicular to contact line) as shown in Fig. 6B. Generally these cracks form due to the stress developed inside the film during final stages of evaporation. From Fig. 6, it appears that for anisotropic particles, the shape and local ordering determine the crack pattern.11 Remarkably, in case of higher aspect ratio ellipsoids, the major axis of the particle is parallel to the crack direction and therefore, the crack direction can be used as an indicator of particle ordering as shown in Fig. 6C. Assuming that the particles are of comparable polydispersity, the reason for the observed difference in the microscopic and macroscopic features is probably due to the fact that in higher aspect ratio particles the direction dependent interactions are more dominant.
Conclusions
We have presented a simple single step bottom-up approach for the self-assembly of nano-ellipsoids into three dimensional ordered structures. To this end, we exploited the use of a combination of vertical deposition technique and the DLVO interactions. One of the main advantages of the proposed strategy is that the self-assembled structures are formed in the absence of any external field – either magnetic or electric. The model colloidal dispersion used in this investigation consisted of hematite nano-ellipsoids dispersed in aqueous medium at different pH conditions. This enables us to investigate the effect of particle geometry, aspect ratio and DLVO interactions on the microscopic and macroscopic structure of the dry films obtained via vertical deposition. The arrangement of particles in the film – either random (amorphous) or long range order can be tuned via the control of particle surface charge. A three-dimensional ordering of ellipsoids across the entire width and thickness of the film is observed when the particles are highly charged. The nano-ellipsoids in the film are arranged in a closed packed manner with particles major axis parallel to the contact line. In the closed packed structures, particles are arranged in different lattice structures as a result of the same overall DLVO interaction energy. When the particles are weakly charged, the elliptical particles in the film are randomly oriented giving an amorphous particulate film. We also show that the particle arrangement has an effect on macroscopic appearance of the films – cracks are observed in films with ordered arrangement of ellipsoids, whereas there is no indication of any crack in the amorphous films. The aspect ratio of the particles is found to have an effect on the local arrangement of particles and the crack pattern that result. While our work clearly demonstrate the role of interactions on the arrangement of anisotropic particles in the films formed after vertical deposition, control over crack formation, effect of particle concentration and evaporation rate needs to be further investigated to take full advantage of the proposed controlled evaporation driven self-assembly technique.Notes and references
- P. Vukusic and J. R. Sambles, Nature, 2003, 424, 852–855 CrossRef CAS PubMed.
- H. Benisty, S. Olivier, C. Weisbuch, M. Agio, M. Kafesaki, C. M. Soukoulis, Q. Min, M. Swillo, A. Karlsson, B. Jaskorzynska, A. Talneau, R. Moosburger, M. Kamp, A. Forchel, R. Ferrini, R. Houdre and U. Oesterle, IEEE J. Quantum Electron., 2002, 38, 770–785 CrossRef CAS.
- S. Y. Chou, M. S. Wei, P. R. Krauss and P. B. Fischer, J. Appl. Phys., 1994, 76, 6673–6675 CrossRef CAS PubMed.
- E. C. Dickey, O. K. Varghese, K. G. Ong, D. Gong, M. Paulose and C. A. Grimes, Sensors, 2002, 2, 91–110 CrossRef CAS PubMed.
- D. Schneider, P. J. Beltramo, M. Mattarelli, P. Pfleiderer, J. Vermant, D. Crespy, M. Montagna, E. M. Furst and G. Fytas, Soft Matter, 2013, 9, 9129–9136 RSC.
- P. J. Beltramo, D. Schneider, G. Fytas and E. M. Furst, Phys. Rev. Lett., 2014, 113, 205503 CrossRef.
- J. D. Forster, J.-G. Park, M. Mittal, H. Noh, C. F. Schreck, C. S. O’Hern, H. Cao, E. M. Furst and E. R. Dufresne, ACS Nano, 2011, 5, 6695–6700 CrossRef CAS PubMed.
- T. Ding, K. Song, K. Clays and C.-H. Tung, Adv. Mater., 2009, 21, 1936–1940 CrossRef CAS PubMed.
- X. C. Jiang, Q. H. Zeng, C. Y. Chen and A. B. Yu, J. Mater. Chem., 2011, 21, 16797–16805 RSC.
- V. R. Dugyala, S. V. Daware and M. G. Basavaraj, Soft Matter, 2013, 9, 6711–6725 RSC.
- M. Mittal and E. M. Furst, Adv. Funct. Mater., 2009, 19, 3271–3278 CrossRef CAS PubMed.
- A. A. Shah, H. Kang, K. L. Kohlstedt, K. H. Ahn, S. C. Glotzer, C. W. Monroe and M. J. Solomon, Small, 2012, 8, 1551–1562 CrossRef CAS PubMed.
- N. D. Denkov, O. D. Velev, P. A. Kralchevsky, I. B. Ivanov, H. Yoshimura and K. Nagayama, Nature, 1993, 361, 26 CrossRef PubMed.
- J. J. Crassous, A. M. Mihut, E. Wernersson, P. Pfleiderer, J. Vermant, P. Linse and P. Schurtenberger, Nat. Commun., 2014, 5, 1–7 Search PubMed.
- D. Kim, W. D. Kim, M. S. Kang, S.-H. Kim and D. C. Lee, Nano Lett., 2015, 15, 714–720 CrossRef CAS PubMed.
- B. Madivala, J. Fransaer and J. Vermant, Langmuir, 2009, 25, 2718–2728 CrossRef CAS PubMed.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Nature, 1997, 389, 827–829 CrossRef CAS.
- D. J. Norris, E. G. Arlinghaus, L. Meng, R. Heiny and L. E. Scriven, Adv. Mater., 2004, 16, 1393–1399 CrossRef CAS PubMed.
- Z. Zhou and X. S. Zhao, Langmuir, 2004, 20, 1524–1526 CrossRef CAS.
- P. Jiang, J. F. Bertone, K. S. Hwang and V. L. Colvin, Chem. Mater., 1999, 11, 2132–2140 CrossRef CAS.
- S.-L. Kuai, X.-F. Hu, A. Haché and V.-V. Truong, J. Cryst. Growth, 2004, 267, 317–324 CrossRef CAS PubMed.
- Z. Zhou and X. S. Zhao, Langmuir, 2005, 21, 4717–4723 CrossRef CAS.
- Y.-H. Ye, F. LeBlanc, A. Haché and V.-V. Truong, Appl. Phys. Lett., 2001, 78, 52–54 CrossRef CAS PubMed.
- A. Hartsuiker and W. L. Vos, Langmuir, 2008, 24, 4670–4675 CrossRef CAS PubMed.
- P. Born, S. Blum, A. Munoz and T. Kraus, Langmuir, 2011, 27, 8621–8633 CrossRef CAS PubMed.
- L. Wang and X. S. Zhao, J. Phys. Chem. C, 2007, 111, 8538–8542 CAS.
- J.-M. Meijer, F. Hagemans, L. Rossi, D. V. Byelov, S. I. R. Castillo, A. Snigirev, I. Snigireva, A. P. Philipse and A. V. Petukhov, Langmuir, 2012, 28, 7631–7638 CrossRef CAS PubMed.
- T. A. Shastry, J. W. T. Seo, J. J. Lopez, H. N. Arnold, J. Z. Kelter, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, Small, 2013, 9, 45–51 CrossRef CAS PubMed.
- T. Sugimoto, Y. Wang, H. Itoh and A. Muramatsu, Colloids Surf., A, 1998, 134, 265–279 CrossRef CAS.
- M. Ocana, M. P. Morales and C. J. Serna, J. Colloid Interface Sci., 1999, 212, 317–323 CrossRef CAS PubMed.
- V. R. Dugyala and M. G. Basavaraj, J. Phys. Chem. B, 2015, 119(9), 3860–3867 CrossRef CAS PubMed.
- C. Nobile, L. Carbone, A. Fiore, R. Cingolani, L. Manna and R. Krahne, J. Phys.: Condens. Matter, 2009, 21(26), 264013 CrossRef PubMed.
- V. R. Dugyala and M. G. Basavaraj, Langmuir, 2014, 30, 8680–8686 CrossRef CAS PubMed.
- P. Schiller, S. Kruger, M. Wahab and H.-J. Mögel, Langmuir, 2011, 27, 10429–10437 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Document contains measurement of zeta potential of hematite particle as a function pH, results of peeling experiment that demonstrate self-assembly of particles across the cross section of the film and DLVO potential calculations. See DOI: 10.1039/c5ra09632d |
| This journal is © The Royal Society of Chemistry 2015 |