Magnetic mesoporous iron oxide/carbon aerogel photocatalysts with adsorption ability for organic dye removal
Yi-Feng Lin* and
Chia-Yu Chang
Department of Chemical Engineering and R&D Center for Membrane Technology, Chung Yuan Christian University, Chungli, Taoyuan 32023, Taiwan. E-mail: yflin@cycu.edu.tw
First published on 20th June 2014
Abstract
Magnetic photocatalysts with strong Rhodamine B (RhB) dye adsorption ability, i.e., tri-functional mesoporous composite γ-Fe2O3/α-Fe2O3/carbon aerogel (CA) structures, are successfully developed in this study. The removal capacity of RhB dyes of the as-prepared mesoporous structures is increased from 83.5% to 91% under visible light irradiation. The mesoporous structures can also be separated by an external magnetic field to avoid further separation steps, such as centrifugation. This work demonstrates that the as-prepared tri-functional mesoporous composite γ-Fe2O3/α-Fe2O3/CA structures have great potential in wastewater treatments.
Photocatalytic nanomaterials, such as TiO2,1 ZnO2 and CdS,3 are well-known nanomaterials in environmental applications.4–6 In photocatalytic applications, pollutants first diffuse to the photocatalyst surface in the solution and then are adsorbed on the photocatalyst surface. Next, the pollutants are decomposed by the photocatalysts under light irradiation. The adsorption step is very important for the photocatalytic application. If the pollutants are not adsorbed by the photocatalysts, the decomposition process of the pollutants cannot proceed. As a result, photocatalysts with adsorption ability are better for photocatalytic applications. On the other hand, the photocatalysts must be separated from the pollutant solution by external steps, such as centrifugation, after the decomposition process. Consequently, magnetic photocatalysts are a potential material to avoid external separation steps, as magnetic photocatalysts can be separated from the pollutant solution by an external magnetic field.
Iron oxides are well-known magnetic building blocks with three different crystalline phases: magnetite (Fe3O4), maghemite (γ-Fe2O3) and hematite (α-Fe2O3). Consequently, relevant iron oxide nanostructures, such as nanoparticles,7 nanowires,8 nanorods,9 nanocubes,10 nanotubes11 and mesoporous structures,12 have been successfully prepared in the literature. Among the three crystalline phases of iron oxide, Fe3O4 and γ-Fe2O3 are ferrimagnetic materials,13,14 indicating that they can be separated from the pollutant solution by an external magnetic field. However, they do not possess photocatalytic properties, so they cannot decompose the pollutants but can adsorb them in the solution. On the other hand, α-Fe2O3 possesses the opposite properties from those of Fe3O4 and γ-Fe2O3. α-Fe2O3 is a well-known visible light photocatalyst,15 and it can decompose pollutants under visible light irradiation. However, α-Fe2O3 is an antiferrimagnetic building block,16 indicating that it cannot be separated from the pollutant solution by an external magnetic field, and further separation steps are needed.
In this work, magnetic photocatalysts with strong adsorption properties, i.e., tri-functional mesoporous iron oxide/CA structures, are successfully fabricated by a facile hydrothermal process. The as-prepared mesoporous composite γ-Fe2O3/α-Fe2O3/CA structures have ferrimagnetic properties due to their γ-Fe2O3 component, and they also have photocatalytic properties because of their α-Fe2O3 component. Furthermore, the as-fabricated mesoporous γ-Fe2O3/α-Fe2O3/CA structures are also demonstrated to show strong RhB dye adsorption. As a result, the as-prepared tri-functional γ-Fe2O3/α-Fe2O3/CA structures have a large removal capacity for RhB dyes under visible light irradiation and can be separated from the solution by an external magnetic field, as shown in Scheme 1. This result indicates that the tri-functional γ-Fe2O3/α-Fe2O3/CA structures have great potential in wastewater treatment, such as organic dye removal.
Ferrous sulfate (FeSO4), hexamethylenetetramine (HMTA) and DI water were used to prepare the iron oxide nanorods. The synthesis of carbon aerogel (CA) was based on the procedure described in our previous report.17 Different amounts of RhB dyes were dissolved in DI water to form different concentration of RhB dyes for the RhB dyes adsorption experiments. The adsorption capacity as a function of time is carried out using an initial RhB dye concentration of 8 ppm and it reaches equilibrium after 12 h. As result, the adsorption experiments are all run for 12 h. The initial concentration of RhB dyes of 16 ppm was used for the photodegradation experiments under visible light irradiation using 500 W xenon lamp at room temperature for 12 h. The iron oxide nanorods were prepared using a facile hydrothermal process. HMTA and FeSO4 were dissolved in 60 ml of deionized water. After the solution was stirred for 2 h, the solution mixture was transferred to a 100 ml Teflon-lined autoclave. The autoclave was subsequently heated at 180 °C for 24 h. After cooling to room temperature, the product was washed several times with ethanol and then dried at 50 °C for 24 h. The resulting powders were further annealed at 400 °C under atmospheric conditions for 7 h to form the γ-Fe2O3/α-Fe2O3 nanorods (400N). For the preparation of mesoporous γ-Fe2O3/α-Fe2O3/CA structures, FeSO4 and CA were added to 60 ml of deionized water and stirred for 24 h. The resulting black precipitates were further dried at 50 °C for 24 h. The as-obtained black powders and HMTA were added to 60 ml of DI water and stirred for 2 h. The solution was then transferred to a 100 ml Teflon-lined autoclave, and the autoclave was heated at 180 °C for 24 h. After cooling to room temperature, the product was washed with ethanol and then dried at 50 °C for 24 h. After drying, the as-obtained products were further annealed at 400 °C under atmospheric conditions for 7 h to form the mesoporous γ-Fe2O3/α-Fe2O3/CA structures (CA-400).
The crystalline structures and surface morphologies of the as-prepared γ-Fe2O3/α-Fe2O3 nanorods (400N) without the addition of CA in the reactants and mesoporous γ-Fe2O3/α-Fe2O3/CA structures (CA400) samples were investigated by XRD and FESEM, as shown in Fig. 1. The XRD patterns of the 400N and CA400 samples are shown in Fig. 1(c) and (d). Also included in Fig. 1(a) and (b) are the diffraction patterns of reference γ- and α-Fe2O3 crystals, respectively. All diffraction peaks of the 400N and CA400 samples appear to fit well with the diffraction peaks of reference α-Fe2O3 crystals.
However, after carefully examining the diffraction peak at 34° to 38°, the peak at approximately 35.6° in the patterns of the 400N and CA400 samples fits well with the peak for the reference γ-Fe2O3 crystal (inset in Fig. 1), indicating that both the 400N and CA400 samples are composite γ-/α-Fe2O3 materials. The rod-like shape of the 400N samples is observed in Fig. 1(e), indicating the formation of composite γ-/α-Fe2O3 nanorods with specific surface area of 27.7 m2 g−1 in the 400N samples. However, a spherical-like shape is observed in Fig. 1(f) with the addition of CA. The addition of CA restricts the growth of nanorods to form the mesoporous composite γ-/α-Fe2O3/CA structures with specific surface area of 551 m2 g−1 in the CA400 samples.
The adsorption of pollutants on the surface of a photocatalyst is the first step in the photodegradation of pollutants using a photocatalyst. After adsorption, the pollutants are decomposed by the photocatalyst under light irradiation. As a result, the strong adsorption ability of the photocatalyst is also very important for further photocatalytic applications. The adsorption ability of the 400N and CA400 samples is determined from the plots of the RhB dye removal capacity (Qe) vs. the equilibrium concentration of RhB dyes (Ce), as shown in Fig. 2. For the CA400 samples, the removal capacity of RhB dyes reaches a stable value of approximately 145 mg g−1 in the dark (CA400-D), indicating the as-prepared CA400 samples have a high capacity to adsorb RhB dye due to their large specific surface area. However, the capacity to remove RhB dye is increased to 165 mg g−1 when using CA-400 samples under visible light irradiation (CA400-L). The increased removal capacity of the RhB dyes results from the photocatalytic behavior of the CA400 samples, especially from their α-Fe2O3 component. On the other hand, the removal capacity of RhB dyes by the 400N samples under visible light irradiation is slightly larger than in the dark, but it is still substantially smaller than that of the CA400 samples. This is because the specific surface area of the 400N samples (27.7 m2 g−1) is much smaller than that of the CA400 samples (551 m2 g−1). The adsorption data (400N-D and CA400-D samples) were further used to fit the Langmuir isotherm model, and the fitting coefficients (R2) of the 400N-D and CA400-D samples are 0.995 and 0.994, respectively, indicating that the Langmuir model fit the adsorption data of both the 400N and CA400 samples well. This result indicates that a monolayer of RhB dye is adsorbed onto the 400N and CA400 samples, which is beneficial for further photodegradation. When visible light irradiates the 400N and CA400 samples, the monolayer of adsorbed RhB dye is easily decomposed due to its direct contact with the photocatalyst surface. If a multilayer of RhB dye is adsorbed on the photocatalyst surface, some of the RhB dye cannot be decomposed by the photocatalyst due to the lack of contact with the photocatalyst surface. As a result, the behavior of monolayer adsorption assists the photocatalytic process.
The reuse of the as-prepared CA400 samples was evaluated over three recycles, as shown in Fig. 3. The percentage of RhB dye recovered after the third cycle was approximately 98%, which is the same as the percentage recovered after the first use. This result indicates that the as-prepared CA400 samples are reusable for RhB dye removal. Thus, the as-prepared CA400 samples are potential materials for use in RhB dye removal in industrial applications.
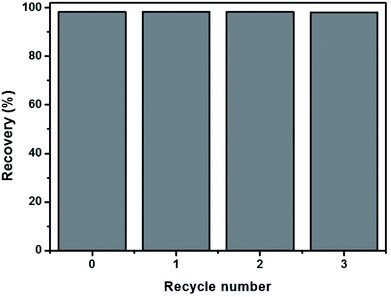 | ||
| Fig. 3 Recycle curves of RhB dye removal using the CA-400 sample; the initial RhB dye concentration was 8 ppm. | ||
The removal percentages of RhB dye by the 400N and CA400 samples in the dark (400N-D and CA400-D) and under visible light irradiation (400N-L and CA400-L) are shown in Fig. 4 with an initial RhB dye concentration of 16 ppm. The removal percentages of the RhB dye are approximately 1%, 2%, 83.5% and 91% for 400N-D, 400N-L, CA400-D and CA400-L samples, respectively. Under both conditions, the CA400 samples have greater RhB dye removal capacities than those of 400N samples due to their larger specific surface areas. For the CA400 samples, the removal percentage of RhB dye increased from 83.5% in the dark to 91% under visible light irradiation. The increase in the removal of RhB dye under visible light irradiation results from the α-Fe2O3 component in the CA400 samples. α-Fe2O3 materials are anti-ferrimagnetic metal oxides and well-known visible light photocatalysts. Unlike α-Fe2O3 materials, γ-Fe2O3 materials do not possess photocatalytic ability, but they are well-known ferrimagnetic metal oxides. Therefore, we prepared composite mesoporous γ-Fe2O3/α-Fe2O3 structures with ferrimagnetic and photocatalytic properties. Because the as-prepared CA400 samples are mesoporous γ-Fe2O3/α-Fe2O3/CA structures with strong RhB dye adsorption, it means the CA400 samples possess tri-functional properties, ferrimagnetic, photocatalytic and adsorption properties. When the tri-functional CA400 samples are dispersed in RhB dye solution under visible light irradiation (Scheme 1), the RhB dye is first adsorbed by the CA400 samples due to their strong adsorption property, and then the adsorbed RhB dye is further decomposed by the CA400 photocatalyst. Afterwards, the CA400 samples can be easily separated from the solution by an external magnetic field due to their ferrimagnetic property, as shown in Scheme 1. As a result, the as-prepared mesoporous γ-Fe2O3/α-Fe2O3/CA structures have great potential in wastewater treatment, such as organic dye removal.
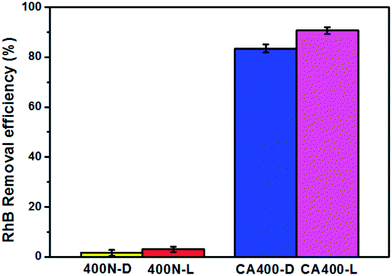 | ||
| Fig. 4 The removal efficiency of RhB dye by 400N-D, 400N-L, CA400-D and CA400-L, with an initial RhB dye concentration of 16 ppm. | ||
Conclusions
In conclusions, tri-functional mesoporous γ-Fe2O3/α-Fe2O3/CA structures with magnetic, adsorption and photocatalytic properties are successfully prepared using a facile hydrothermal process. The as-prepared tri-functional materials possess strong adsorption ability of RhB dyes and form a monolayer of RhB dyes on the materials surface, which benefits further photocatalytic process. The RhB dyes removal capacity using CA400 samples are increased after visible light irradiation due to their α-Fe2O3 composition. The CA400 samples can be easily separated from the solution by an external magnetic field due to their ferrimagnetic properties. As a result, the as-synthesized tri-functional mesoporous γ-Fe2O3/α-Fe2O3/CA structures are a great potential in wastewater treatments, such as organic dye removal.Notes and references
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed
.
- Y. Wang, X. Li, N. Wang, X. Quan and Y. Chen, Sep. Purif. Technol., 2008, 62, 727–732 CrossRef CAS PubMed
.
- C. Li, J. Yuan, B. Han and W. Shangguan, Int. J. Hydrogen Energy, 2011, 36, 4271–e4279 CrossRef CAS PubMed
.
- H. Y. Wu, F. K. Shieh, H. M. Kao, Y. W. Chen, J. R. Deka, S. H. Liao and K. C. W. Wu, Chem.–Eur. J., 2013, 19, 6358–6367 CrossRef CAS PubMed
.
- I. J. Kuo, N. Suzuki, Y. Yamauchi and K. C. W. Wu, RSC Adv., 2013, 3, 2028–2034 RSC
.
- F. K. Shieh, C. T. Hsiao, H. M. Kao, Y. C.Sue, K. W. Lin, C. C. Wu, X. H. Chen, L. Wan, M. H. Hsu, J. R. Hwu, C. K. Tsung and K. C. W. Wu, RSC Adv., 2013, 3, 25686–25689 RSC
.
- A.-H. Lu, E. L. Salabas and F. Schth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed
.
- X. Wen, S. Wang, Y. Ding, Z.-L. Wang and S. Yang, J. Phys. Chem. B, 2005, 109, 215–220 CrossRef CAS PubMed
.
- S. Lian, E. Wang, Z. Kang, Y. Bai, L. Gao, M. Jiang, C. Hu and L. Xu, Solid State Commun., 2004, 129, 485–490 CrossRef CAS PubMed
.
- D. Kim, N. Lee, M. Park, B.-H. Kim, K. An and T. Hyeon, J. Am. Chem. Soc., 2009, 131, 454–455 CrossRef CAS PubMed
.
- C.-J. Jia, L.-D. Sun, Z.-G. Yan, L.-P. You, F. Luo, X.-D. Han, Y.-C. Pang, Z. Zhang and C.-H. Yan, Angew. Chem., Int. Ed., 2005, 44, 4328–4333 CrossRef CAS PubMed
.
- Y.-F. Lin and J.-L. Chen, RSC Adv., 2013, 3, 15344–15349 RSC
.
- O. Bretcanu, S. Spriano, E. Verne, M. Coisson, P. Tiberto and P. Allia, Acta Biomater., 2005, 1, 421–429 CrossRef CAS PubMed
.
- S. Morup, J. Magn. Magn. Mater., 2003, 266, 110–118 CrossRef CAS
.
- Y. Shi, H. Li, L. Wang, W. Shen and H. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4800–4806 CAS
.
- C. Frandsen and S. Morup, J. Magn. Magn. Mater., 2003, 266, 36–48 CrossRef CAS
.
- S.-H. Hsu, Y.-F. Lin, T.-W. Chung, T.-Y. Wei, S.-Y. Lu, K.-L. Tung and K.-T. Liu, Sep. Purif. Technol., 2013, 109, 129–134 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |



