Charge transfer luminescence of hafnates under synchrotron vacuum ultraviolet excitation†
De-Yin Wangab,
Chien-Hao Huangb,
Bing-Ming Chengc,
Teng-Ming Chen*b and
Yu-Hua Wanga
aDepartment of Physical Science and Technology, Lanzhou University, Lanzhou 730000, China
bDepartment of Applied Chemistry, National Chiao Tung University, Hsinchu 30010, Taiwan. E-mail: tmchen@mail.nctu.edu.tw; Fax: +886-35723764; Tel: +886-35731695
cNational Synchrotron Radiation Research Center, Hsinchu 30076, Taiwan
First published on 18th June 2014
Abstract
Under synchrotron vacuum ultraviolet excitation, three different hafnates (BaHf(PO4)2, BaHf(BO3)2 and BaHfSi3O9) show self-activated luminescence with peaks ranging from 275–335 nm, which is attributed to the charge-transfer luminescence from the hafnate (HfO68−) group. Upon doping Eu2+ into BaHfSi3O9, host sensitization of Eu2+ emission via hafnate intrinsic emission is demonstrated.
Oxide compounds consisting of transition-metal ions without d-electrons, such as titanates, vanadates, molybdates, niobates and tungstates, are known to luminesce.1 The mechanism responsible for the observed luminescence is the ligand-to-metal charge transfer (LMCT).1 The corresponding charge transfer absorptions in these compounds are found to be generally situated in the ultraviolet region.1 Recent studies on LMCT from transition-metal ions have been extended to zirconates, in which Zr4+ with a d0 configuration plays an important role.2,3 Unlike these transition-metal containing compounds mentioned above, the charge transfer (CT) absorptions of zirconates are found to locate at a much higher energy level,2,3 and generally it requires the excitation light to have an energy in the vacuum ultraviolet region (VUV; E > 50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1; λ < 200 nm) so that zirconates can be excited into their charge transfer state.2,3 Hf4+ belongs to the same fourth group element as that of Ti4+ and Zr4+, thus CT luminescence is expected from hafnates as well. However, after examining the existing literature, only a few works on the luminescence of hafnates were reported, among which most are focused on the X-ray excited luminescence,4–7 partially for the purpose of developing phosphor for medical X-ray imaging applications. Examples are BaZr1−xHfPO4,4 Li2HfO3,5 HfP2O7,6 Hf1−xZrxGeO4.7 From these works, we aware that hafnates can give luminescence upon X-ray excitation, but information regarding their absorption is still rare. The lack of studies for the charge transfer luminescence from hafnates is presumably due to the technique and method of conventional UV excitations that are unable to excite hafnates, as is the case for zirconates. With the advancement of synchrotron radiation, it is possible to investigate the photoluminescence properties of phosphors in VUV region. Investigation of spectroscopic properties of a material in the VUV region provides important and instructive information needed for engineering VUV phosphors used for PDPs and Hg-free lamp. In this paper, we investigated the CT luminescence of three different hafnates (BaHf(PO4)2, BaHf(BO3)2 and BaHfSi3O9) which have HfO68− group in their crystal structure using synchrotron VUV excitation. In addition, Eu2+ has been widely used as the luminescent center for phosphor applied in lighting and displays,8 with an aim to develop new VUV phosphor, we also studied the VUV-excited luminescence of Eu2+-doped BaHfSi3O9 and demonstrated the unprecedented host sensitization of Eu2+ emission using hafnate intrinsic emission.
000 cm−1; λ < 200 nm) so that zirconates can be excited into their charge transfer state.2,3 Hf4+ belongs to the same fourth group element as that of Ti4+ and Zr4+, thus CT luminescence is expected from hafnates as well. However, after examining the existing literature, only a few works on the luminescence of hafnates were reported, among which most are focused on the X-ray excited luminescence,4–7 partially for the purpose of developing phosphor for medical X-ray imaging applications. Examples are BaZr1−xHfPO4,4 Li2HfO3,5 HfP2O7,6 Hf1−xZrxGeO4.7 From these works, we aware that hafnates can give luminescence upon X-ray excitation, but information regarding their absorption is still rare. The lack of studies for the charge transfer luminescence from hafnates is presumably due to the technique and method of conventional UV excitations that are unable to excite hafnates, as is the case for zirconates. With the advancement of synchrotron radiation, it is possible to investigate the photoluminescence properties of phosphors in VUV region. Investigation of spectroscopic properties of a material in the VUV region provides important and instructive information needed for engineering VUV phosphors used for PDPs and Hg-free lamp. In this paper, we investigated the CT luminescence of three different hafnates (BaHf(PO4)2, BaHf(BO3)2 and BaHfSi3O9) which have HfO68− group in their crystal structure using synchrotron VUV excitation. In addition, Eu2+ has been widely used as the luminescent center for phosphor applied in lighting and displays,8 with an aim to develop new VUV phosphor, we also studied the VUV-excited luminescence of Eu2+-doped BaHfSi3O9 and demonstrated the unprecedented host sensitization of Eu2+ emission using hafnate intrinsic emission.
Samples of undoped BaHf(PO4)2, BaHf(BO3)2, BaHfSi3O9 and Eu2+-doped BaHfSi3O9 were prepared in powder form by using high temperature solid state reaction methods. The phase purity of all samples was checked by using powder X-ray diffraction (XRD) analysis. The photoluminescence (PL) and photoluminescence excitation (PLE) spectra were measured at BL03A beamline of the National Synchrotron Radiation Research Center (NSRRC) in Hsinchu, Taiwan. Samples synthesis details and experimental setup for photoluminescence spectra are provided as Supplementary Information.
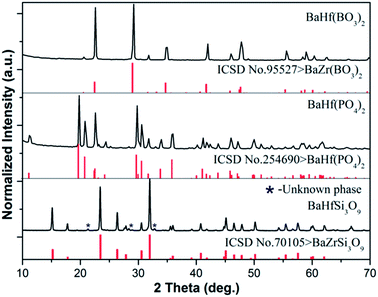
Fig. 1 shows the XRD patterns of BaHf(BO3)2, BaHf(PO4)2 and BaHfSi3O9 synthesized in present work. Since no reported standard XRD pattern is available for BaHf(BO3)2 and BaHfSi3O9, their phase purity was identified by using the XRD data of the corresponding zirconate as a reference. The XRD patterns of the three hafnates are in good agreement with the reference data reported in ICSD no. 254690 for BaHf(PO4)2, ICSD no. 95527 for BaZr(BO3)2 and ICSD no. 70105 for BaZrSi3O9, with the exception of an unknown phase marked by an asterisk (I% < 4%, 2θ = 21.1°, 28.3° and 32.8°) observed in BaHfSi3O9 (Fig. 1). This observation indicates that each hafnate retained the same crystal structure as that of its corresponding zirconate. Previous structural study on BaHf(PO4)2 (ref. 9), BaZr(BO3)2 (ref. 10) and BaZrSi3O9 compounds (ref. 11) has shown that a Zr–O octahedron unit exists in their crystal structures. Due to the similar XRD patterns between the zirconate and hafnate, we suggest the hafnium is six-coordinated in all of the three hafnates under investigation.
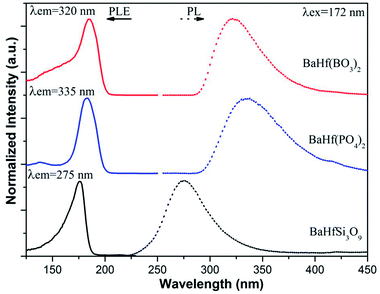
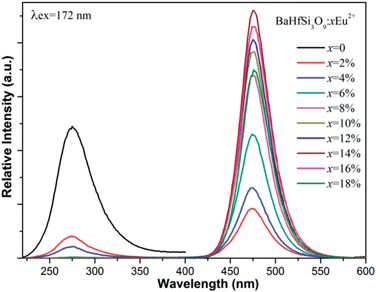
Fig. 2 shows the normalized PLE and PL spectra of BaHf(BO3)2, BaHf(PO4)2 and BaHfSi3O9. Under excitation at 172 nm, BaHf(PO4)2, BaHf(BO3)2 and BaHfSi3O9 show broad emission with maxima at 335 nm, 320 nm and 275 nm, respectively. We set the excitation light at 172 nm because in practice the available VUV light source (Xe/Ne excimer discharge) gives the main emission at this wavelength. This observation suggests that hafnates may serve as UV-emitting phosphors and find their new applications in transcription of repair enzymes and treatment of skin diseases such as psoriasis et al.12 In analogy to the case of the VUV-excited luminescence of zirconates,1–3 the observed broad emission is assigned to the CT luminescence from the hafnate (HfO68−) group, which can be explained as the recombination of the excited electron on Hf4+ ions with hole left on O2− ligand during the CT absorption process. In reality, it is considered that the charge transfer transition doesn't involve in transferring one electron but does involve a considerable reorganization of the charge density distribution around the metal ion.13 The PLE spectra for these three hafnates obtained by monitoring the emission maximum of each compound are all composed of an intense band in the range of 125–200 nm with peaks at 185 nm, 184 nm and 176 nm for BaHf(PO4)2, BaHf(BO3)2 and BaHfSi3O9, respectively, which is attributed to the charge transfer transition from ligand O2− to metal Hf4+, i.e., electron is transferred from the 2p orbital of the surrounding O2− ions into the empty 5d orbital of Hf4+. After electron transfer to Hf4+, the hole appears to be distributed over ligands around the Hf3+ ion. In the reverse process, the radiative recombination of electron at Hf3+ (formed after electron transfer) with the hole localized on O2− gives rise to the observed charge transfer luminescence. According to the Forster–Dexter energy transfer theory,14 resonant energy transfer from a sensitizer to an acceptor happens when certain conditions are met, i.e., (1) a considerable spectral overlap between the sensitizer's emission and acceptor's absorption, and (2) a reasonable distance between the sensitizer and acceptor.14 In our previous work, Eu2+ has been shown to have a broad 4f–5d absorption ranging from 280–400 nm in BaHfSi3O9–Eu2+,15 while in present work the hafnate group in BaHfSi3O9 gives broad UV emission with maximum at 275 nm under VUV excitation (see Fig. 2). This fact provides the beneficial conditions for energy transfer from hafnate group to Eu2+ based on the Forster–Dexter energy transfer theory, and implies that the CT emission from hafnates could be utilized to sensitize Eu2+ emission in BaHfSi3O9–Eu2+ upon VUV excitation. To see whether sensitization of Eu2+ emission by hafnates CT luminescence occurs, we measured the PL spectra of BaHfSi3O9 doped with various concentrations of Eu2+ (see Fig. 3) under 172 nm excitation. When 2% Eu2+ is introduced into BaHfSi3O9, it is found that the CT luminescence from hafnate is greatly reduced. With further increasing the Eu2+ doping concentration, accompanied by an increase of Eu2+ 5d–4f emission at 474 nm, the charge transfer luminescence from hafnate decreases further, demonstrating that energy transfer from hafnate group to Eu2+ indeed occurs via hafnate CT luminescence. The CT luminescence from hafnate group is almost quenched due to such energy transfer when the Eu2+ doping concentration is increased to 6%. With increasing Eu2+ doping concentration, more Eu2+ ions are distributed in the host matrix, thus the energy transfer probability between hafnate group and Eu2+ is increased, resulting in the observation that the CT luminescence decreases while the Eu2+ emission increases with increasing Eu2+ dopant concentration. When Eu2+ doping concentration is increased to 14%, Eu2+ emission starts to decrease due to concentration quenching.
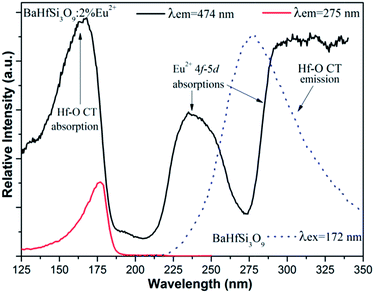
Fig. 4 presents the PL excitation spectra of a 2% Eu2+ doped sample detected by monitoring the two emission bands located at 275 nm and 474 nm together with the PL spectrum of host matrix BaHfSi3O9. The PLE spectrum detecting Eu2+ emission and that detecting hafnate CT luminescence has a similar absorption band below 200 nm assigned to Hf4+–O2− charge transfer absorption. The small shift for the Hf4+–O2− charge transfer absorption band observed in the PL excitation spectra probably due to the emission grating blazes differently at 275 nm and 474 nm. The presence of the Hf4+–O2− CT absorption band in the excitation spectrum monitored within the Eu2+ emission further indicates that the energy transfer from hafnate group to Eu2+ ions via Hf4+–O2− CT luminescence has occurred. The two additional bands above 200 nm in the PLE spectrum monitoring the Eu2+ emission (that are not observed in the PL spectrum monitoring Hf4+–O2− CT luminescence) are assigned to the 4f–5d absorption of Eu2+ due to crystal field splitting. The perfect spectral overlap between Hf–O CT emission and Eu2+ 4f–5d absorption provides favorable conditions for energy transfer from hafnate group to Eu2+. Process of Hf4+–O2− CT luminescence and energy transfer from hafnate intrinsic emission to Eu2+ is schematically illustrated in Scheme S1 (ESI†).
In summary, photoluminescence of three different hafnium-containing compounds, BaHf(PO4)2, BaHf(BO3)2 and BaHfSi3O9, has been studied using synchrotron vacuum ultraviolet excitation. These three hafnates show absorption in the region of 125–200 nm with a maximum close to 180 nm, which results from charge transfer from O2− ligand to Hf4+ metal. Under VUV excitation at 172 nm, BaHf(PO4)2, BaHf(BO3)2 and BaHfSi3O9 show self-activated luminescence peaking at 335 nm, 320 nm and 275 nm, which is attributed to the charge-transfer luminescence from the hafnate (HfO68−) group, and can be rationalized as the recombination of the excited electron on Hf4+ ions with the hole left on the O2− ligand created during the charge transfer absorption. Upon doping Eu2+ into the host matrix of BaHfSi3O9, sensitization of Eu2+ emission via Hf4+–O2− charge transfer luminescence occurs, which is evidenced by the significant decreasing of Hf4+–O2− charge transfer luminescence and increasing of Eu2+ emission intensity with increasing Eu2+ doping concentration, along with the presence of similar excitation band for the Eu2+ doped and undoped BaHfSi3O9.
Acknowledgements
This work was supported by National Science Council of Taiwan through Contract nos. NSC 100-2811-M-009-068, NSC 101-2113-M-009-021-MY3 and in part by the Fundamental Research Funds for the Central Universities (LZUJBKY-2014-36) and Open Project of Lanzhou University Key Laboratory for Magnetism and Magnetic Materials of the Ministry of Education (LZUMMM2014012).Notes and references
- G. Blasse, Struct. Bonding, 1980, 42, 1 CrossRef CAS.
- M. Kaneyoshi, J. Lumin., 2006, 121, 102 CrossRef CAS PubMed.
- D. Y. Wang, L. L. Wang, T. J. Lee, T. M. Chen and B. M. Cheng, J. Electrochem. Soc., 2011, 158, J377 CrossRef CAS PubMed.
- C. R. Miao and C. C. Torardi, J. Solid State Chem., 2000, 155, 229 CrossRef CAS.
- Y. V. Baklanova, A. V. Ishchenko, T. A. Denisova, L. G. Maksimova, B. V. Shulgin, V. A. Pustovarov and L. V. Viktorov, Opt. Mater., 2012, 34, 1037 CrossRef CAS PubMed.
- L. H. Brixner and G. Blasse, J. Solid State Chem., 1991, 91, 390 CrossRef CAS.
- P. M. Lambert, P. S. Bryan, G. S. Jarrold and C. M. Towers, U.S. Pat., 5173611, 1992.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Mater. Sci. Eng., R, 2010, 71, 1 CrossRef PubMed.
- K. Popa, D. Bregiroux, R. M. Konings, T. Gouder, A. F. Popa, T. Geisler and P. E. Raison, J. Solid State Chem., 2007, 180, 2346 CrossRef CAS PubMed.
- V. Hornebecq, P. Gravereau, J. P. Chaminade and E. Lebraud, Mater. Res. Bull., 2002, 37, 2165 CrossRef CAS.
- K. Iwasaki, Y. Takahashi, H. Masai and T. Fujiwara, Opt. Express, 2009, 17, 18054 CrossRef CAS PubMed.
- S. Okamoto, R. Uchino, K. Kobayashi and H. Yamamoto, J. Appl. Phys., 2009, 106, 013522 CrossRef PubMed.
- L. van Pieterson and A. Meijerink, J. Alloys Compd., 2000, 300–301, 426 CrossRef CAS.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836 CrossRef CAS PubMed.
- D. Y. Wang, Y. C. Wu and T. M. Chen, J. Mater. Chem., 2011, 21, 18261 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Samples synthesis details and experimental setup for photoluminescence spectra, schematic process of Hf–O CT luminescence and energy transfer from hafnate instrince emission to Eu2+. See DOI: 10.1039/c4ra03481c |
| This journal is © The Royal Society of Chemistry 2014 |