Light-induced wide range color switching of liquid crystal blue phase doped with hydrogen-bonded chiral azobenzene switches†
Ouyu Jina,
Dengwei Fua,
Jie Weia,
Huai Yangb and
Jinbao Guo*a
aCollege of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: guojb@mail.buct.edu.cn
bDepartment of Materials Science and Engineering, College of Engineering, Peking University, Beijing 100871, China
First published on 19th June 2014
Abstract
A novel class of hydrogen-bonded chiral azobenzene switches was developed to induce an optically tunable liquid crystal blue phases (LC-BPs), which leads to the stabilization of the LC-BPs for a wider temperature range and is capable of a reversible phototuning of the reflection color of BP I over the visible wavelength region.
Blue phases (BPs) that self organize into a three-dimensional periodic cubic lattice are of great interest due to their unique characteristics such as no birefringence but selective reflection of circularly polarized light, and are proven to be a promising materials platform for self-assembling tunable photonic materials, fast display, mirrorless lasers and so on.1 BPs normally appear in the temperature range between chiral nematic liquid crystal (N*-LC) phases and isotropic phases. Three thermodynamically distinct BPs are observed during cooling from the isotropic state: BP III, BP II and BP I. BP I and BP II are believed to be body-centered cubic and simple cubic structure, respectively. While BP III is macroscopically amorphous with a local cubic lattice in the director field.2 However, practical application of BPs has been restricted by the narrow stable temperature range, and more efforts have been, therefore, directed to overcome this obstacle, such as polymer stabilization, nanoparticles and special molecular design.3 Meanwhile, increasing attention has been paid to tuning photonic band gaps (PBGs) by applying external stimuli, such as electrical field, temperature and optical field.4,5
Among these options, optically tunable BPs behavior can be efficiently achieved with the help of trans–cis photoisomerization of azobenzene.5 However, a very few BP systems on wide optical tuning of PBGs have been reported. Q. Li et al. demonstrated a wide optical switching of PBGs of BPs accompanied with phase transition from BP II to BP I in BP-LCs doped with a chiral azobenzene switch.5d A light-controlled PBGs of BPs system composed of a series of a chiral azobenzene-dimers was recently reported by H. Yang et al., and light-induced BP I to N* phase transition was also observed in this BP material system.5e However, to our knowledge, a wide optical tuning of PBGs in a single and stable BP has not been observed until now.
Here we firstly report a new kind of hydrogen-bonded chiral azobenzene switches (H-bonded CAS) as facile self-organized soft materials, which can efficiently enable BPs to response to light as well as to stabilize BPs. More importantly, a stable BP I without any phase transitions can be reversible tuned by UV/Vis light irradiation over the visible region across red, green, and blue wavelengths. We note that H-bonded effect may have a positive influence for the stabilization of BPs and photoisomerization of azobenzene may be responsible for wide optical tuning characteristics.
As is shown in Fig. 1, binaphthylazobenzene molecule (BNAzo) is used as the proton acceptor; the detailed synthesis procedure and characterization were provided in Fig. S1 of ESI;† while 4-methylhexanoic acid (MHA) and octanoic acid (OCA) are chosen to act as proton donors. The H-bonded molecular switches (BNAzo–MHA and BNAzo–OCA) are assembled by the method shown in ESI.† The H-bonded CAS combined with bent-shape dopant (TBOA–MBIN) and S811 (Fig. S2†) were doped into commercially available nematic LCs (SLC 1717) to induce BPs as demonstrated in ESI.† The FT-IR spectroscopy and variable-temperature FT-IR spectroscopy were used to distinguish the existence and thermal stability of H-bonds (Fig. 2 and S3†). FT-IR analysis confirm the presence of H-bonding obtained by self-assembly process and good thermal stability across a temperature range of 30 °C to 110 °C. Fig. 1 also shows the optimized molecular structures of BNAzo–MHA and BNAzo–OCA before and after UV irradiation obtained by molecular simulation based on Gaussian 03 calculations at the B3LYP/6-31G (d) level. Additionally, the molecular aspect ratios (L/D) of these two H-bonded CAS, defined as the ratio of the molecular length (L) to the molecular diameter (D), are also provided in Fig. 1.
 | ||
| Fig. 1 The chemical structures of H-bonded CAS and their optimized structures of trans and cis form of BNAzo–MHA and BNAzo–OCA obtained by Gaussian 03 calculations at the B3LYP/6-31G (d) level. | ||
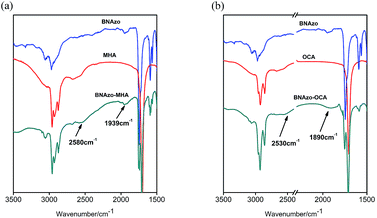 | ||
| Fig. 2 FT-IR spectra of the H-boned CAS and their precursors (a): BNAzo–MHA, BNAzo, MHA; (b): BNAzo–OCA, BNAzo, OCA. | ||
By using Cano wedge measurement, we can find that the helical twisting power (HTP) values of both BNAzo–MHA and BNAzo–OCA in N*-LCs decrease during UV light irradiation and heating process (see Fig. S5 and Table S1†). The change of geometrical configuration from trans to cis isomer of BNAzo contributed to the HTP change by UV light irradiation as discussed later. Meanwhile, for the HTP change with variation in temperature, it is possible due to the associated change in the orientation parameter of the molecule as a result of weakened or strengthened H-bonded interaction of H-bonded CAS in the host LCs.6 These observations also correspond to the data analysis of circular dichroism spectroscopy and UV/Vis spectroscopy of H-bonded CAS in solvent as demonstrated in Fig. 3 and S4.† Additionally, the HTP value of BNAzo–OCA is higher than that of BNAzo–MHA and the change of HTP of BNAzo–OCA is also larger after UV irradiation as shown in Table S1.† These results coincide with the molecular aspect ratio we listed in Fig. 1, which is one of key factors influencing HTP in previous work.7
 | ||
| Fig. 3 UV/Vis absorption and CD spectra of H-bonded CAS in 1,4-dioxane under UV irradiation (a): BNAzo–MHA; (b): BNAzo–OCA. | ||
To get an understanding of how H-bonded CAS affects the temperature range of BPs, we prepared a series of LC samples as listed in Table 1. It should be noted that, in order to keep almost the same chiral strength for all the samples (verified by Fig. S6 in ESI†), we decreased the concentrations of S811 when increased the amount of H-bonded CAS. Our results clearly show that H-bonded CAS could serve to stabilize BPs by comparing the doped samples A1–A4 and undoped sample A0. Additionally, increasing the H-bonded CAS content resulted in broadened temperature ranges of BPs. For example, by increasing BNAzo–MHA content from 2.5 to 3.5 wt%, the BP range increased from 16.7 °C to 21.0 °C in samples A1 and A2. As a typical sample, the phase sequence of the sample A2 on cooling process is from the isotropic phase (I), BP I to N*-LC with a BP temperature range of ≈21 °C, which is larger than that of a typical BP LCs and is very close to room temperature. These observations can be explained due to the fact that, similar to HTP in Ch-LCs, the chiral strength of both BNAzo–MHA and BNAzo–OCA increased during cooling process, which is of benefit to keeping a high chirality and correspondingly helps to stabilize BPs in LC system. Additionally, our experimental results also demonstrate that, despite the HTP value of BNAzo–OCA is higher; a same increase in the BNAzo–OCA from 2.5 to 3.5% in sample A3 and A4 resulted in a rather small increase in BP temperature, i.e., from 15.4 °C to 20.2 °C. This may be attributed to the following two points: on one hand, the change of HTP (or chiral strength) of BNAzo–MHA is a little larger than that of BNAzo–OCA as temperature is decreased as listed in Table S1.† On the other hand, as shown in Fig. 1, there exists a branched terminal group in MHA, which makes BNAzo–MHA much bent than BNAzo–OCA as shown in Fig. 1, this result is very similar to the polymer-induced stabilization effect of BPs associated with a branched structure of the side alkyl group in the polymer chain and also observed our BP-LC systems.3b,8
| Sample no. | Compositiona/wt% | Transition temperature/°C | |||||
|---|---|---|---|---|---|---|---|
| H-bonded CAS | S811 | SLC 1717 | N*-BP | BP I | ΔTb | ||
| a Each sample contains 20 wt% TBOA–MBIN.b ΔT: BP temperature range. | |||||||
| A0 | None | 30 | 70 | 18.6 | 31.9 | 13.3 | |
| A1 | BNAzo–MHA | 2.5 | 27 | 70.5 | 21.8 | 38.5 | 16.7 |
| A2 | BNAzo–MHA | 3.5 | 25 | 71.5 | 26.8 | 47.8 | 21.0 |
| A3 | BNAzo–OCA | 2.5 | 27 | 70.5 | 25.1 | 40.5 | 15.4 |
| A4 | BNAzo–OCA | 3.5 | 25 | 71.5 | 28.9 | 49.1 | 20.2 |
In order to evaluate the effectiveness of the H-bonded CAS on the optical tuning of reflection band of BP, we fabricate a sample A5 with 3.5 wt% BNAzo–OCA and 30 wt% S811 in 66.5 wt% SLC 1717. Therefore, the initial BP reflection band moved to blue region as a result of increased concentration of S811. It is well-established that the reflection wavelength will appear discontinuous jump and shift approximately 50–80 nm if the phase transition happens from BP II to BP I.4b We note particularly that, temperature dependence of the reflection wavelength, shown in Fig. 4, clearly shows that the reflection wavelength of samples A5 and A3 exhibited a continuous and little change with a decrease in temperature. Therefore, we confirm that there is no BP II to BP I transition in our samples due to the highly chirality doped with bent dopants as reported in previous studies.9
 | ||
| Fig. 4 Reflection wavelength of BP I in samples A5 and A3 as a function of temperature with a cooling rate of 0.5 °C min−1. | ||
Here the optical switching of BP doped with H-bonded CAS was performed at 48 °C and therefore the thermal effect can be eliminated. As is shown in Fig. 5a, on UV irradiation (365 nm, 10 mW cm−2), the reflection bands are found to have a red-shift from 473 nm to 642 nm. After 8 s of irradiation, the reflection band of BP I no longer shifted and stayed at 642 nm. On the other hand, when irradiated by visible light (450 nm, 15 mW cm−2) it recovered to the original state. Fig. 5b shows POM images for the BP I of sample A5 at 48 °C by UV light irradiation. As the irradiation time was increased, the various BP platelet colors of blue, green, and red were observed, which is corresponding to the real LC cell images with a continuous shift of reflection wavelength under UV irradiation as shown in Fig. 5c. Herein, to provide a direct comparison between regions with and without irradiation, a photomask was used so that only the center of the LC cell was illuminated as shown in Fig. 5c. Furthermore, we note that no phase transitions including BP II to BP I or BP I to Ch were found during the phototuning process in our system, which means that optical tuning process only happens in BP I. This result may have the following explanations. First, according to the temperature dependence of the reflection wavelength of BP-LCs, we confirm no BP II exists in our samples as demonstrated before; second, due to high chirality, the transition from BP I to Ch also don't happen even if we added UV irradiation. From the above discussion, we can clearly see that the reflection wavelength of BP I was reversible tunable over the visible region across red, green, and blue wavelengths.
Fig. 6 shows the illustrations of BP-LCs doped with H-bonded CAS and the corresponding phototuning mechanism of double twisted cylinder (DTC) structure before and after light irradiation. Here the photoresponsive behavior is derived from the proton acceptor (BNAzo) of H-bonded CAS, in which BNAzo has two azo linkages resulting in reversible trans–cis isomerization of azo configurations on light irradiation. As demonstrated in Fig. S5,† due to the disorganization effect of cis-isomer with a bent shape, the HTP value decreases in N*-LC after light irradiation. Herein, similar to N*-LC, the photoinduced trans–cis isomerization of BNAzo in BP-LCs doped with H-bonded CAS was also found to decrease the chirality of BPs and correspondingly increase the lattice constant of DTC structure of BP-LCs as shown in Fig. 6, which was verified by the above spectra data of Fig. 5. Furthermore, the reflection band of BP-LCs undergoes a red shift due to the trans–cis isomerization upon UV irradiation and the restoration of the reflection will occur when the cis isomer turns back to the trans form under 450 nm light.
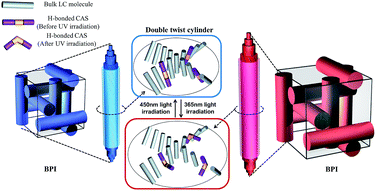 | ||
| Fig. 6 The possible illustration of H-bonded CAS doped into the BPs and partial enlargement of the double twist cylinder before and after irradiation. | ||
In summary, we employ a new kind of H-bonded CAS to form an optically tunable BP material system with a wide tuning range, in which the reflection wavelength of BP I was reversibly tuned through the visible region across red, green, and blue wavelengths, and light-induced behavior only happens in stable BP I and no phase transitions appeared during the light-controlled process. Meanwhile, the temperature range of BPs is expanded from 13.3 to 21.0 °C. The possible mechanism of these results may be attributed to the changes of chirality induced by both UV/Vis light irradiation and temperature. This work points out a way to design chiral self-organized soft materials suitable for optically addressable BP displays and other optical device application.
Acknowledgements
This research was supported by National Natural Science foundation (Grant no. 51373013, 51173013, 50903004) and Beijing Young Talents Plan (YETP0489).Notes and references
- (a) J. Yan, L. Rao, M. Jiao, Y. Li, H.-C. Cheng and S.-T. Wu, J. Mater. Chem., 2011, 21, 7870–7877 RSC; (b) S. Yokoyama, S. Mashiko, H. Kikuchi, K. Uchida and T. Nagamura, Adv. Mater., 2006, 18, 48–51 CrossRef CAS; (c) A. Mazzulla, G. Petriashvili, M. A. Matranga, M. P. De Santo and R. Barberi, Soft Matter, 2012, 8, 4882–4885 RSC; (d) W. Cao, A. Munoz, P. Palffy-Muhoray and B. Taheri, Nat. Mater., 2002, 1, 111–113 CrossRef CAS PubMed.
- (a) D. C. Wright and N. D. Mermin, Rev. Mod. Phys., 1989, 61, 385–432 CrossRef CAS; (b) K. Higashiguchi, K. Yasui and H. Kikuchi, J. Am. Chem. Soc., 2008, 130, 6326–6327 CrossRef CAS PubMed; (c) O. Henrich, K. Stratford, M. E. Cates and D. Marenduzzo, Phys. Rev. Lett., 2011, 106, 107801 CrossRef CAS.
- (a) H. J. Coles and M. N. Pivnenko, Nature, 2005, 436, 997–1000 CrossRef CAS PubMed; (b) H. Kikuchi, M. Yokota, Y. Hisakado, H. Yang and T. Kajiyama, Nat. Mater., 2002, 1, 64–68 CrossRef CAS PubMed; (c) W. He, G. Pan, Z. Yang, D. Zhao, G. Niu, W. Huang, X. Yuan, J. Guo, H. Cao and H. Yang, Adv. Mater., 2009, 21, 2050–2053 CrossRef CAS; (d) F. Castles, F. Day, S. Morris, D. Ko, D. Gardiner, M. Qasim, S. Nosheen, P. Hands, S. Choi and R. Friend, Nat. Mater., 2012, 11, 599–603 CrossRef CAS PubMed; (e) E. Karatairi, B. Rožič, Z. Kutnjak, V. Tzitzios, G. Nounesis, G. Cordoyiannis, J. Thoen, C. Glorieux and S. Kralj, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 81, 041703 CrossRef.
- (a) S.-Y. Lu and L.-C. Chien, Opt. Lett., 2010, 35, 562–564 CrossRef CAS PubMed; (b) H. Choi, H. Higuchi and H. Kikuchi, Appl. Phys. Lett., 2011, 98, 131905 CrossRef PubMed.
- (a) A. Chanishvili, G. Chilaya, G. Petriashvili and P. J. Collings, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 71, 1–5 CrossRef; (b) H.-C. Jeong, K. V. Le, M.-J. Gim, S.-T. Hur, S.-W. Choi, F. Araoka, K. Ishikawa and H. Takezoe, J. Mater. Chem., 2012, 22, 4627–4630 RSC; (c) H.-Y. Liu, C.-T. Wang, C.-Y. Hsu, T.-H. Lin and J.-H. Liu, Appl. Phys. Lett., 2010, 96, 121103 CrossRef PubMed; (d) T. H. Lin, Y. Li, C. T. Wang, H. C. Jau, C. W. Chen, C. C. Li, H. K. Bisoyi, T. J. Bunning and Q. Li, Adv. Mater., 2013, 25, 5050–5054 CrossRef CAS PubMed; (e) X. Chen, L. Wang, C. Li, J. Xiao, H. Ding, X. Liu, X. Zhang, W. He and H. Yang, Chem. Commun., 2013, 49, 10097–10099 RSC; (f) W. R. Browne and B. L. Feringa, in Molecular Switches, ed. B. L. Feringa and W. R. Browne, Wiley-VCH, Weinheim, 2nd edn, 2011, p. 121 Search PubMed.
- A. Takahashi, V. A. Mallia and N. Tamaoki, J. Mater. Chem., 2003, 13, 1582–1587 RSC.
- Y. Xie, D. Fu, O. Jin, H. Zhang, J. Wei and J. Guo, J. Mater. Chem. C, 2013, 1, 7346–7356 RSC.
- (a) Y. Shi, X. Wang, J. Wei, H. Yang and J. Guo, Soft Matter, 2013, 9, 10186–10195 RSC; (b) J. Guo, Y. Shi, X. Han, O. Jin, J. Wei and H. Yang, J. Mater. Chem. C, 2013, 1, 947–957 RSC.
- (a) B. Li, W. He, L. Wang, X. Xiao and H. Yang, Soft Matter, 2013, 9, 1172–1177 RSC; (b) Z. Kutnjak, C. W. Garland, c. G. Schatz, P. J. Collings, C. J. Booth and J. W. Goodby, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1996, 53, 4955–4963 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02680b |
| This journal is © The Royal Society of Chemistry 2014 |

