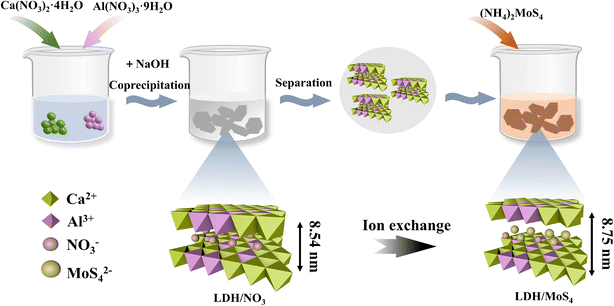
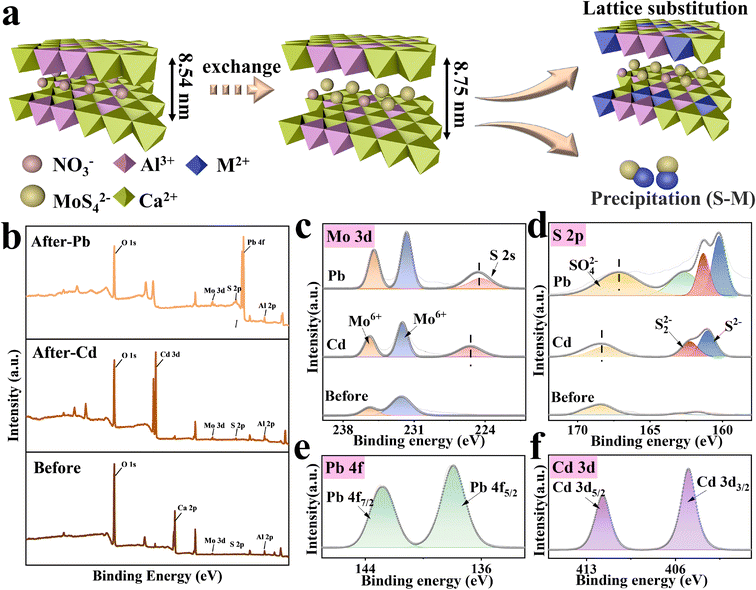
Unveiling the dual roles of the intercalation of [MoS4]2− clusters in boosting heavy metal capture by Ca–Al layered double hydroxide†
Fangshu
He
a,
Zhihui
Yang
a,
Feiping
Zhao
*a,
Eveliina
Repo
b,
Weichun
Yang
a,
Qi
Liao
a,
Mengying
Si
 a,
Bin
Zou
a and
Zhang
Lin
a,
Bin
Zou
a and
Zhang
Lin
 a
a
aChinese National Engineering Research Center for Control & Treatment of Heavy Metal Pollution, Institute of Environmental Engineering, School of Metallurgy and Environment, Central South University, School of Metallurgy and Environment, Changsha 410083, Hunan, China. E-mail: feiping.zhao@csu.edu.cn; Tel: +86 138 7584 6982
bDepartment of Separation Science, School of Engineering Science, Lappeenranta-Lahti University of Technology LUT, Yliopistonkatu 34, FI-53850, Finland
First published on 29th November 2022
Abstract
Heavy metals such as Pb(II) and Cd(II) are ubiquitously present in aquatic and soil environments and pose a critical threat to the environment and human health. Herein, we report a strategy to prepare high-performance adsorbent materials by assembling [MoS4]2− groups between the lamellae of CaAl-LDHs (LDH/MoS4) for Pb(II) and Cd(II) removal. The assembling of LDH/MoS4 was achieved by a facile coprecipitation and anion exchange method. The intercalation with [MoS4]2− not only expanded the interlayer spacing but also created more active sites for the capture of the targeted heavy metals. The obtained LDH/MoS4 nanocomposites demonstrated enormous adsorption capacities of 1202.1 and 678.3 mg g−1 for Pb(II) and Cd(II), respectively. The excellent adsorption of Pb(II) and Cd(II) by LDH/MoS4 could be attributed to the following: Pb(II) and Cd(II) can form strong M–S covalent bonds with the [MoS4]2− clusters, and Cd(II) can easily undergo lattice substitution with Ca(II) on the layer of LDHs owing to the similar ionic radius of Ca(II) and Cd(II). Moreover, density functional theory (DFT) calculations further verified the adsorption mechanisms of Pb(II) and Cd(II) sorption. Altogether, its facile fabrication and rapid and ultrahigh adsorption efficiencies demonstrate the importance of LDH/MoS4 in the simultaneous removal of multiple heavy metal ions in practical applications.
Environmental significanceThe study reported one concept for fabrication of [MoS4]2− clusters intercalated into Ca–Al layered double hydroxide (LDH/MoS4) serving as an efficient adsorbent for heavy metal removal. The proposed materials exhibit superior removal efficiency toward Pb(II) (1202.1 mg g−1) and Cd(II) (678.3 mg g−1). Removal mechanisms involve Pb–S bonding for Pb(II) and lattice substitution for Cd(II). The study suggested that the intercalation with [MoS4]2− clusters plays significant roles in heavy metal remediation from wastewater and contaminated soil. |
1. Introduction
With the rapid development of socio-economic and living standards, increasing levels of heavy metals (e.g., lead and cadmium) in the environment have become one of the most serious dangers to humans and the aquatic environment due to their toxicity and carcinogenic properties.1,2 Therefore, there is an urgent demand for heavy metal pollutants to be removed from water bodies. Various remediation techniques, including chemical precipitation, flocculation, electrolysis, ion exchange, and membrane separation, have been developed in recent years to remove lead and cadmium cations from wastewater;3–6 however, these procedures are costly and ineffective. The adsorption method has been considered as one of the most frequently used and affordable water remediation techniques due to its simple operation, environmental friendliness, and low cost.7 Over the past decades, numerous adsorbents, such as biochar,8 hydroxyapatite,9 nano zero-valent iron,10 and clay minerals,11 have been developed for the removal of heavy metals from aqueous solutions. However, these materials still suffer from the drawbacks of low efficiency, slow kinetics, and poor selectivity and stability. Thus, it is crucial to develop efficient functional adsorbents with high affinity toward heavy metals.Unlike cationic clay minerals, layered double hydroxides (LDHs) are anionic clay compounds12,13 that consist of stacked cationic layers and counter anions between the interlayers. Generally, the host layers are two-dimensional structural layers of nanomaterial with the general formula [M2+1−xM3+x(OH)2]x(An−)x/n·mH2O, where M2+ and M3+ serve as divalent and trivalent cations while An− represents the counter anion.14,15 LDHs have been extensively used in the removal of heavy metals due to their high ion-exchange capacity, large specific surface area, easy synthesis procedure, and environmental friendliness.14,16 However, the removal of heavy metals utilizing pristine LDHs is hampered due to the positively charged laminates. Significantly, the types of layered bimetals and the ions inserted between the layers that constitute LDHs are tunable and designable.17–19 Owing to these attractive features, many functionalities have been proposed to enhance the adsorptive capacities of LDHs. Huang et al. tuned the surface charge density of NiFe-LDHs through iron valence change to enhance the performance in adsorption of Cr(VI).20 According to Zhu et al.,21 Zn–Al LDH could be intercalated with amino trimethyl phosphonic acid (ATMP) as an efficient adsorbent for Cu(II) and Pb(II) (42.02 mg g−1 for Cu(II) and 84.06 mg g−1 for Pb(II)) in wastewater. It is an effective alternative to improve the adsorption properties of LDHs by intercalation of anions with high affinity toward heavy metals into the interlayers of LDHs.
According to Pearson's hard–soft Lewis acid–base principles, sulfide-based materials possess superior affinity toward many Lewis acidic soft heavy metal cations.22 Recently, several novel metal sulfides such as [MoS2], [WS2], [K2xMnxSn3−xS6],23,24 chalcogenide-based clusters13 and aerogels25 have been successfully applied for highly efficient removal of heavy metals from contaminated gas, water, and soil. Among them, chalcogenide clusters such as [Sn2S6]4−, [SnS4]4−, and [MoS4]2− exhibit robust sulfur-active sites and pore structures13,22,26 possessing a great potential in heavy metal capture. However, owing to the mobility of nanoclusters, chalcogenide clusters cannot be utilized directly in practical application. A suitable host for chalcogenide clusters is also essential when used for adsorption. The tunable and ion exchangeable properties of the interlayers of LDHs make them an ideal candidate for hosting sulfur clusters. There are some reports focusing on the insertion of [MoS4]2− cluster into the interlayer of LDH and demonstrating extremely high performance, such as Fe–MoS4 LDH,27 Mn–MoS4 LDH,28 and MgAl–MoS4 LDH.29 However, their adsorption mechanism is still concerned with the efficient selectivity of the [MoS4]2− cluster to heavy metals so that focus on the dual adsorption sites of layers and interlayer anions has been rarely explored; they usually only pay attention to the interlayer anions, but not make full use of the large number of adsorption sites on the layers. Herein, we report the intercalation of [MoS4]2−, a typical chalcogenide cluster, into the interlayers of CaAl-LDHs by a facile ultrasonic-assisted ion exchange method (see Fig. 1) and evaluate its removal efficiencies toward Pb(II) and Cd(II) from aqueous solutions. In this setting, the combination of LDHs and [MoS4]2− could bring considerable benefits: (i) the interlayers of the positively charged layers of CaAl-LDHs provide host channels for the immobilization of [MoS4]2− nanoclusters; (ii) the intercalation modification with [MoS4]2− as an interlayer substance not only enlarges the specific surface area but also expands the interlayer distance of CaAl-LDHs, thus accelerating the diffusion of heavy metals in the interlayer spacing; (iii) the soft Lewis basic sulfur clusters immobilized in the interlayers of CaAl-LDHs possess a strong affinity toward soft Lewis acidic heavy metal ions, forming a stable complex (S–M). Thus, the intercalation of [MoS4]2− into LDHs is expected to be a win–win approach to make up for the shortcomings of both parties, especially for the enhancement of the adsorptive performance for heavy metals. The as-prepared hybrid of CaAl-LDHs and [MoS4]2− (LDH/MoS4) exhibited unprecedented removal efficiencies of Pb(II) and Cd(II). Although previous studies explored the mechanisms of Pb(II) and Cd(II) removal by sulfur-containing groups through material characterization, it was not clear why the sorption capacities of Pb(II) and Cd(II) differ in these systems, and the microscopic mechanisms of their interlayer space in LDHs were not illustrated.21 Therefore, the adsorbent–sorbate interaction mechanisms and the mutual effects of each party of the hybrid on adsorption were fully explored by both density functional theory (DFT) calculations and experimental characterization.
2. Experimental section
2.1. Materials
All reagents were purchased from Sinopharm and were used as received without further purification. Stock solutions of 2000 mg L−1 were prepared by dissolving the appropriate amounts of Pb(II) and Cd(II) nitrate salts (analytic grade) in deionized water. Working solutions of Pb(II) and Cd(II) ranging from 1 to 1500 mg L−1 were prepared by diluting the stock solutions. The solution pH was adjusted by using 0.1 M NaOH and 0.1 M HNO3.2.2. Synthesis of LDH/NO3 and LDH/MoS4 nanocomposites
LDH/NO3 was synthesized by a co-precipitation method.30,31 Briefly, 1.89 g of Ca(NO3)2·4H2O and 0.75 g of Al(NO3)3·9H2O were dissolved in 100 mL of deionized water, obtaining bimetallic mixed solution A. Meanwhile, 8.8 g of NaOH was dissolved in 50 mL of deionized water to obtain alkali solution B. The pH of the mixture was maintained at about 11, and the mixture was stirred at 800 rpm for 30 min at room temperature. After that, the filtrate was filtered, washed, and then dried in a vacuum at 60 °C. The solid material was ground to finally obtain a white dried powder.LDH/MoS4 was fabricated via an ion exchange reaction using (NH4)2MoS4 as the source of [MoS4]2− clusters. Briefly, 0.5 g of CaAl-LDHs were dispersed in 50 mL of 0.2 mmol L−1 (NH4)2MoS4. The mixture was then stirred at ambient temperature for 6 h, resulting in the formation of an orange solution of suspended particles. Finally, the mixture was filtered, washed with alcohol, and dried under vacuum at room temperature, obtaining an orange powder, labeled LDH/MoS4 and preserved in a desiccator. The schematic synthetic strategy of LDH/MoS4 nanocomposites is illustrated in Fig. 1.
2.3. Characterization
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) analyses were performed using a JSM-7900F instrument (JEOL, Japan) to observe the morphology and elemental distribution of the materials. Lattice images of the samples were obtained by high-resolution transmission electron microscopy (HRTEM) using an FEI Talos F200X (USA) instrument at 200 kV. The X-ray diffraction (XRD) spectra of the nanomaterials were characterized by using an X-ray diffractometer (Empyrean, PANalytical B.V., Netherlands) with a Cu Kα irradiation source. The chemical state of the surface of the samples was measured by X-ray photoelectron spectroscopy (XPS) using a Thermo Scientific (China) spectrometer with Kα as the source. The structural transformations and the functional groups of the materials were characterized by Fourier-transform infrared spectroscopy (FTIR) using a Nicolet iS10 spectrometer (Thermo Scientific Corporation, USA) in the spectral range of 4000–400 cm−1. The Brunauer–Emmett–Teller (BET) specific surface area and pore structure were determined using a Tristar®II Plus (USA) by nitrogen adsorption isotherms in the range of relative pressure from 0.0 to 1.0.2.4. Batch experiments
The adsorption of Pb(II) and Cd(II) on LDH/NO3 and LDH/MoS4 was conducted in batch mode to evaluate the influence of condition factors, such as initial pH, agitation time, and initial metal concentration. Typically, 10 mg of adsorbent was mixed with 10 mL of Pb(II) and Cd(II) solutions (dosage of 1 g L−1). The adsorption kinetics of Pb(II) and Cd(II) at an initial concentration of 1500 mg L−1 were investigated at 25 °C with different contact times of 0–1440 min to ensure adsorption equilibrium. The adsorption isotherms were studied at pH 5 with initial concentrations ranging from 50 to 1000 mg L−1 for Pb(II) and Cd(II). At a designed contact time, the mixture was filtered using a 0.22 μm cellulose acetate membrane and diluted with 5% HNO3. The concentrations of Pb(II) and Cd(II) were analyzed using an inductively coupled plasma optical atomic emission spectrometry (ICP-OES) Model Icap 6300 instrument (Thermo Electron Corporation, USA). All the tests were conducted in triplicate, and the adsorption capacities (mg g−1) of adsorbents were calculated as follows: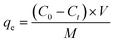 | (1) |
To analyze the competition adsorption of coexisting ions on the adsorption behavior of the LDH/MoS4 for Pb(II) and Cd(II), the competition adsorption experiment was conducted in a quaternary ion system with Cl−(NaCl), NO3− (NaNO3), SO42− (Na2SO4), CO32− (Na2CO3), K+ (KNO3), Mg2+ (Mg(NO3)2), Zn2+ (Zn(NO3)2) and Cu2+ (Cu(NO3)2) as the coexisting ions. Typically, a 0.05 g adsorbent was mixed with 50 mL of solution at 25 °C for 12 h. The initial concentration of each ion was 100 mg L−1. The solution pH remained constant at 5 throughout the sorption periods.
2.5. DFT calculations
All DFT calculations were performed using the Vienna Ab initio Simulation Package (VASP).32 The projector augmented wave (PAW)33 pseudopotential with the PBE generalized gradient approximation (GGA) exchange correlation function was utilized in the computations. The cutoff energy of the plane wave basis set was 500 eV, and a Monkhorst–Pack mesh of 3 × 3 × 1 was used in K-sampling. All structures were spin polarized, and all atoms were fully relaxed with an energy convergence tolerance of 10−5 eV per atom, and the final force on each atom was < 0.01 eV Å−1.The adsorption energy of the reaction intermediates can be computed using eqn (2):
| ΔE = E*+ads − E* − Eads | (2) |
3. Results and discussion
3.1. Characterization of as-prepared materials
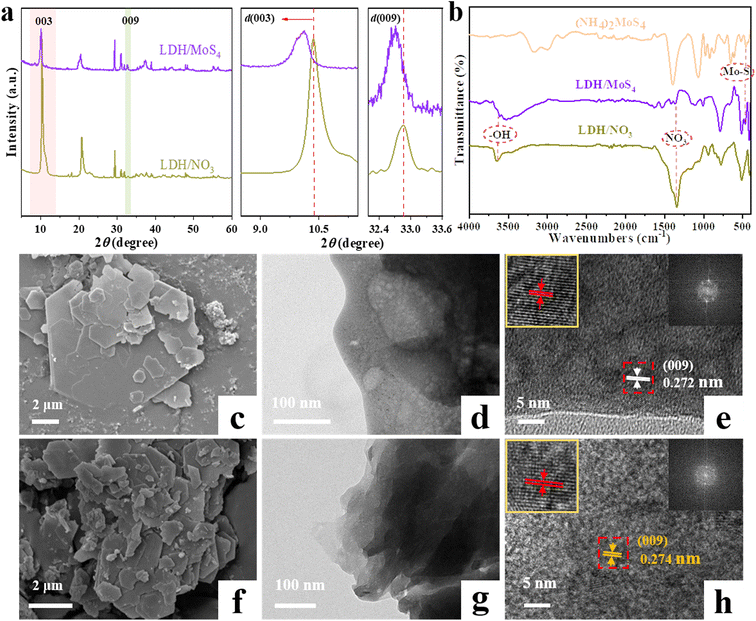
XRD, FTIR, TEM, HRTEM, XPS and other characterization tools were applied to characterize the phases and structures of the as-prepared materials. Remarkably, as shown in Fig. 2, the XRD patterns of LDH/MoS4 show similar results to that of LDH/NO3 without the appearance of new characteristic peaks, which implies that the generation of a new phase did not occur on the materials. Characteristic diffraction peaks at approximately 10.4° and 32.8° are observed for pristine LDH/NO3, which could be assigned to the (003) and (009) planes, respectively.34 However, the diffraction peaks of LDH/NO3 at (003) and (009) shift to lower 2θ angles. Based on the calculation by Bragg's law,35 the d-spacing (d003) of LDH/MoS4 determined by XRD is enlarged to 8.75 Å in comparison with the pristine LDH/NO3 (8.54 Å) (Fig. 2a). Such an enlargement in the basal spacing could be due to the expansion of the unit cell parameter and is in good agreement with the intercalation of larger [MoS4]2− anions than NO3− groups into the interlayer space of LDHs.36 The intercalation of [MoS4]2− into the LDHs results in the color change of the materials from white (LDH/NO3) to orange (LDH/MoS4).The ion exchange of the NO3− groups with [MoS4]2− was further verified by FTIR spectroscopy. Fig. 2b displays the FTIR spectra of LDH/NO3, (NH4)2MoS4, and LDH/MoS4 in the range of 4000–400 cm−1. For the pristine LDH/NO3, the strong band appearing at approximately 1384 cm−1 could correspond to the NO3− of LDH/NO3, indicating the presence of NO3− anions in the interlayers of CaAl-LDHs.26 For the analysis of pure (NH4)2MoS4, the band at approximately 480 cm−1 could be assigned to the Mo–S vibration.37 In the case of LDH/MoS4, the NO3− band at 1384 cm−1 disappeared, while a new sharp peak at approximately 480 cm−1 assigned to the Mo–S bond could be clearly observed, suggesting the successful ion exchange of the NO3− groups with [MoS4]2− during the synthesis process of LDH/MoS4.
The morphologies of LDH/NO3 before and after intercalation of [MoS4]2− were observed by SEM, TEM, and HRTEM. As shown in Fig. 2c and d, the SEM and TEM images of LDH/NO3 demonstrate that the pristine LDH/NO3 has a nanosheet-assembled structure with hexagonal morphology, which is consistent with previously reported MgAl-LDHs.13 The SEM and TEM observations of LDH/MoS4 (Fig. 2f and g) provide evidence of the retention of the platelike two-dimensional morphology after the ion exchange of NO3− groups with [MoS4]2−. This kind of retention of the morphology suggests that the intercalation of [MoS4]2− anions still retains the basic two-dimensional layered structure of LDHs. The HRTEM image of the pristine LDH/NO3 in Fig. 2e exhibits a lattice fringe of 0.272 nm corresponding to the (009) planes of CaAl-LDHs, while the lattice spacing of LDH/MoS4 is observed as 0.274 nm (Fig. 2h). This increase of lattice spacing was consistent with the XRD results. The N2 adsorption–desorption isotherms of LDH/NO3 and LDH/MoS4 are presented in Fig. S1.† Both LDH/NO3 and LDH/MoS4 showed type IV isotherms, which is characteristic of mesoporous materials according to the IUPAC classification.34,38 Moreover, the specific surface areas of LDH/NO3 and LDH/MoS4 were 10.54 and 12.83 mg2 g−1, respectively (Table S1†). LDH/MoS4 possessed a larger surface area than LDH/NO3, which might be attributed to the intercalation modification with [MoS4]2− nanoclusters. Overall, these results confirm the successful intercalation of [MoS4]2− into the interlayers of CaAl-LDHs.
3.2. Adsorption isotherms
To elucidate the adsorption features of the targeted Pb(II) and Cd(II) onto the as-prepared LDH/NO3 and LDH/MoS4, such as adsorption capacities and the interactions between the adsorbent and the adsorbate, two classic isotherm models were employed to fit the experimental data. The Langmuir isotherm supposes that monolayered adsorption occurs on a homogenous surface with uniform adsorption sites. The Freundlich isotherm equation is an empirical model that assumes the adsorption occurs at a heterogeneous surface without a saturation of adsorption sites. As such, the isothermal adsorption models of Langmuir (eqn (3)) and Freundlich (eqn (4)) are given as follows.39,40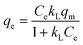 | (3) |
| qe = kfCe1/n | (4) |
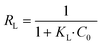 | (5) |
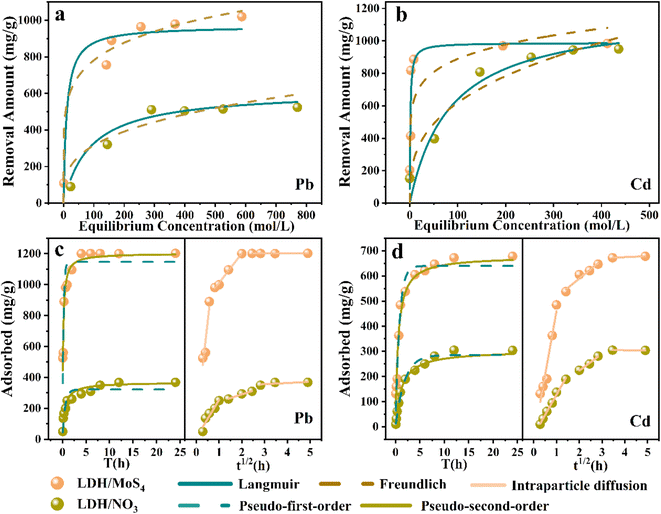
The isotherm non-linear fitting by the two models is illustrated in Fig. 3a and b, and the obtained isotherm constants are listed in Table 1. The results show that LDH/MoS4 exhibited a higher capacity than LDH/NO3. This indicates that the introduction of the [MoS4]2− group considerably enhanced the adsorption capacity, especially for Pb(II). Notably, the Freundlich isotherm model (R2 ≥ 0.96) fits better than the Langmuir isotherm model (R2 ≤ 0.93) for the described data of Pb(II). The well-fitted results with the Freundlich model suggest that the adsorption process of Pb(II) is more likely to be multilayer chemisorption with surface heterogeneous adsorption and active site heterogeneity. In addition, the Kf parameter calculated using the Freundlich model is related to the adsorption capacity; the higher the Kf value is, the easier the adsorption process occurs.41 In this system, the Kf value of LDH/MoS4 for Pb(II) (Kf = 361.5) is much higher than that of LDH/NO3 (Kf = 75.9), indicating that the adsorption of Pb(II) by LDH/MoS4 is more likely to occur.
| Heavy metal | Material | q m,exp (mg g−1) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|---|
| q m,cal (mg g−1) | k L (L mg−1) | R L | R 2 | k f (mg1−n Ln g−1) | n | R 2 | |||
| Pb(II) | LDH/NO3 | 523.8 | 616.6 | 0.011 | 0.604 | 0.909 | 75.9 | 3.23 | 0.764 |
| Cd(II) | LDH/MoS4 | 1020.1 | 968.5 | 0.095 | 0.150 | 0.936 | 361.5 | 5.98 | 0.965 |
| LDH/NO3 | 950.2 | 1177.5 | 0.012 | 0.579 | 0.946 | 129.9 | 2.95 | 0.910 | |
| LDH/MoS4 | 984.6 | 988.1 | 0.808 | 0.020 | 0.882 | 471.6 | 7.26 | 0.681 | |
For the Cd(II) removal data simulation, the Langmuir isotherm model shows a higher correlation coefficient (R2 ≥ 0.88) than the Freundlich model (R2 ≤ 0.68) and the Langmuir curve fitted the experimental data better than the Freundlich curve, indicating that adsorption occurs on a homogeneous surface and the adsorbate is monolayer covered. In the Langmuir isothermal model, RL is an important regularization (calculated by eqn (5)), which is used to verify whether the adsorption in the system under consideration is favorable,42i.e., 0 < RL < 1 (favorable), RL = 0 (irreversible), RL = 1 (linear) or RL > 1 (unfavorable). In this research, Pb(II) achieved sorption RL of 0.15, while Cd(II) sorption achieved RL of 0.02, suggesting that LDH/MoS4 is favorable for both Pb(II) and Cd(II) sorption in the system.
3.3. Adsorption kinetics
The kinetics of Pb(II) and Cd(II) adsorption were investigated with LDH/NO3 and LDH/MoS4 in single-metal nitrate solutions with agitation time from 2 to 1440 min. The pseudo-first-order kinetic model, the pseudo-second-order kinetic model and the intraparticle diffusion model are necessary to investigate adsorption kinetics and further describe the adsorption rate and rate-controlling step.43 In the present study, the pseudo-first-order model (eqn (6)), pseudo-second-order model (eqn (7)) and intraparticle diffusion model (eqn (8)) were selected to fit the experimental data, corresponding to the following equations:44| qt = qe(1 − e−tk1) | (6) |
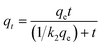 | (7) |
| qt = kit1/2 + C | (8) |
A study of the adsorption kinetics of Pb(II) and Cd(II) on nanocomposites was conducted (Fig. 3c and d), and the experimental data are shown in Table S2.† The results show that the pseudo-first-order adsorption kinetics are less correlated, while the pseudo-second-order model describes the data well (R2 > 0.94), showing that the adsorption rates of Pb(II) and Cd(II) by LDH/MoS4 are dominated by chemisorption control.45 The material adsorption rates of Pb(II) and Cd(II) were faster during the initial 60 min and then gradually decreased until adsorption equilibrium was reached. The adsorption behavior of Pb(II) and Cd(II) on both LDH materials was similar, while the adsorption rate of LDH/MoS4 was faster than that of the precursor LDH/NO3, showing excellent adsorption performance. Significantly, the rate constants of LDH/MoS4 toward Pb(II) and Cd(II) were much higher than those of LDH/NO3, suggesting that the intercalation of [MoS4]2− not only enhanced the adsorption capacities but also accelerated the sorption kinetics.
To further explore the adsorption mechanisms, an intraparticle diffusion kinetic model was fitted to the experimental data,46 and the specific values are presented in Table S3.† The curves of the adsorption process resulted in three linear parts, i.e., three stages of surface diffusion (ka), particle diffusion (kb) and final adsorption equilibrium (kc). Both ka and kb of LDH/MoS4 are higher than those of LDH/NO3, which could explain the rapid adsorption of LDH/MoS4 in the initial stage. This could be attributed to its bigger specific surface area and pore size (Fig. S1†). In addition, the dense stacking of nanosheets has a “narrow” limited space and the [MoS4]2− nanoclusters increase the specific surface and pore channels, which further improve the removal efficiency of heavy metals.47,48
3.4. Competition adsorption of the coexisting ions
Fig. 4 shows the Pb(II) and Cd(II) adsorption capacity of LDH/MoS4 with coexisting anions (Cl−, NO3−, SO42− and CO32−) and cations (K+, Mg2+, Zn2+ and Cu2+) in the solution. The adsorption capacity of LDH/MoS4 is inhibited by 9.3% and 14.89% in the presence of Zn2+ and Cu2+ at two different concentrations. The inhibitory effect of the other two anions is negligible (<5%). However, the coexisting anions could cause little effect on Pb(II) and Cd(II) adsorption capacity with a less than 1% reduction. Hence, LDH/MoS4 shows an outstanding selectivity to Pb(II) and Cd(II) removal.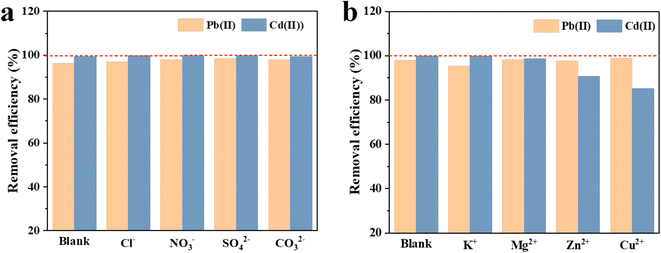 | ||
| Fig. 4 Effect of coexisting anions (a) and cations (b) on the adsorption behavior of LDH/MoS4 for Pb(II) and Cd(II). | ||
3.5. Experimental study on adsorption mechanisms
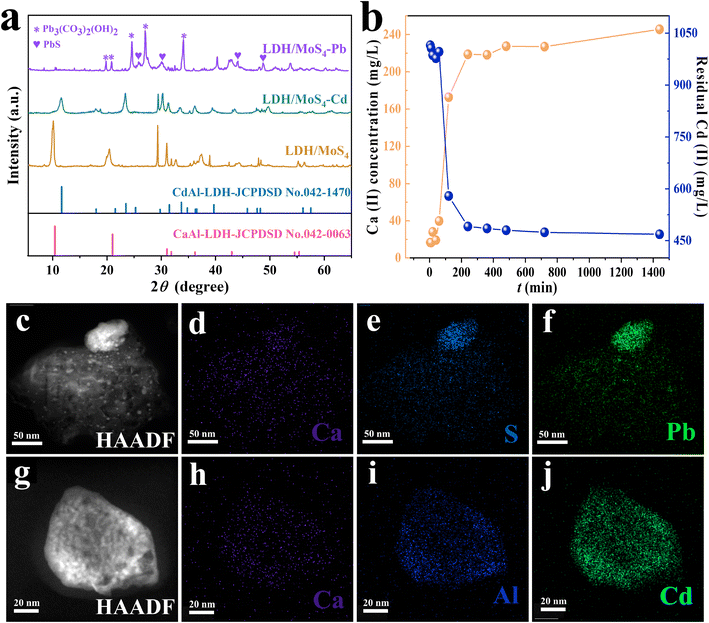
Aiming to shed light on the adsorption mechanisms of LDH/MoS4 toward heavy metals, XRD, XPS, HRTEM and elemental mapping have been conducted. Fig. 5a shows the XRD patterns of LDH/MoS4 before and after metal adsorption. It is evident that the characteristic peaks of LDHs disappeared after the adsorption of Pb(II), revealing that the structure of LDHs is disrupted by possible formation of PbS and Pb3(CO3)2(OH)2 crystal structures. Moreover, the TEM image (Fig. 5c) and the EDS images (Fig. 5d–f and S2†) reveal a clear precipitate on the surface of LDHs, which was a result of Pb–S–Mo precipitation. A higher magnification of EDS mapping, which focuses on the bright spots on the surface of LDH/MoS4, further illustrates the distinct precipitates (Fig. S3†).In contrast, for LDH/MoS4–Cd, the main and basic characteristic peaks of the XRD pattern do not change (Fig. 5a), illustrating that the structure of LDHs is not destroyed upon the adsorption of Cd(II). Moreover, it is clearly seen that LDH/MoS4–Cd has an identical structure to that of CdAl-LDH (JCPDSD No. 042-1470).49 The CdAl-LDH diffraction peak at 10° shifted to a higher value when compared with the original CaAl-LDH due to the fact that Cd(II) (0.97 Å) is smaller than Ca(II) (0.99 Å).50 Furthermore, the larger the ionic radius is, the larger the octahedral volume of LDH. Thus, after Cd(II) adsorption, the lower positive charge density of LDH lamellae led to a lower interlamellar repulsion and a narrower layer spacing. Meanwhile, HRTEM results show that relatively brighter spots were distributed on the LDH/MoS4–Cd in comparison with LDH/MoS4 (Fig. S4†). This could be due to the fact that Cd has a larger atomic mass than Ca and Al.51 It is clear that Cd atoms were well distributed in the lattice fringes of CaAl-LDHs, indicating the successful lattice replacement of Cd into the CaAl-LDHs lattice. Furthermore, as shown in Fig. 5b, the concentration of Ca(II) in the solution increased with time, while the concentration of Cd(II) in the solution decreased, once again demonstrating the successful replacement of the Ca atoms in the LDH lattice by Cd. In contrast, as seen in Fig. S5,† the decline in Pb concentration showed no clear association with Ca concentration, indicating that there is no lattice substitution between Pb and Ca. Moreover, the EDS elemental mapping images of LDH/MoS4 after Cd(II) adsorption were also obtained and the results are shown in Fig. 5h–j. From the EDS analysis, the colorful spots showed that the proportion of Cd (Fig. 5j) is higher than that of Ca (Fig. 5h) due to the lattice replacement of Cd with Ca. Therefore, the XRD, HRTEM, EDS mapping, and leaching results suggest that Cd(II) is immobilized via the lattice substitution of Ca, while Pb(II) is removed by Pb–S bonding to form precipitates.
The adsorbents before and after Pb(II) and Cd(II) adsorption were further analyzed by XPS for their surface chemistry characterization (Fig. 6). In the survey spectra of the LDH/MoS4 material after adsorption, the characteristic peaks of Pb 4f and Cd 3d are clearly observed in Fig. 6b, proving the successful adsorption of Pb(II) and Cd(II) by the composites. In the Mo spectra, the binding energy (BE) peaks at 235.5 eV and 232.2 eV, as shown in Fig. 6c, could correspond to Mo 3d3/2 and Mo 3d5/2, respectively, in accordance with the presence of Mo6+ in the material.52 Moreover, the BE peak of Mo was not shifted significantly after heavy metal adsorption, meaning that no elemental bonding was formed between Mo and the targeted heavy metals. On the other hand, the BE peak for S 2s at 225.6 eV after Pb(II) and Cd(II) adsorption is observed in Fig. 6c, indicating possible sulfide precipitation during the adsorption process of Pb(II) and Cd(II).53 The S 2p XPS spectra are shown in Fig. 6d. The BE peaks at 161.5 eV and 168.5 eV could be attributed to the S 2p3/2 and S 2p1/2 orbitals,24,54 respectively, and the BE peaks of S 2p shifted to a lower direction compared with the original sample, implying that the S in LDH/MoS4 forms a bonding interaction with Pb during the adsorption process. The XPS core lines of the two adsorbed elements presented in Fig. 6e and f show the BE peaks of Pb 4f at 137.33 eV and 142.83 eV assigned to Pb 4f5/2 and Pb 4f7/2, respectively.55 In addition, the BE peaks of Cd 2d at 411.73 eV and 404.98 eV could be assigned to Cd 3d5/2 and Cd 3d3/2, respectively.56 Both of these demonstrate the successful capture of Pb(II) and Cd(II). Moreover, the XPS results (Table S5†) show that the Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O molar ratio of 1
O molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 obtained from the quantitative data is close to the stoichiometric ratio of Ca(OH)2, which is the main material constituting the LDH laminates. The proportion of S
2 obtained from the quantitative data is close to the stoichiometric ratio of Ca(OH)2, which is the main material constituting the LDH laminates. The proportion of S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo after the adsorption of Pb(II) is obviously larger than that after Cd(II) adsorption, which also explains the fact that the interlayer anion [MoS4]2− had a weaker binding for Cd(II) than for Pb(II). The removal mechanisms of the two target heavy metals are shown in Fig. 6a.
Mo after the adsorption of Pb(II) is obviously larger than that after Cd(II) adsorption, which also explains the fact that the interlayer anion [MoS4]2− had a weaker binding for Cd(II) than for Pb(II). The removal mechanisms of the two target heavy metals are shown in Fig. 6a.
3.6. DFT calculations for the adsorption mechanisms
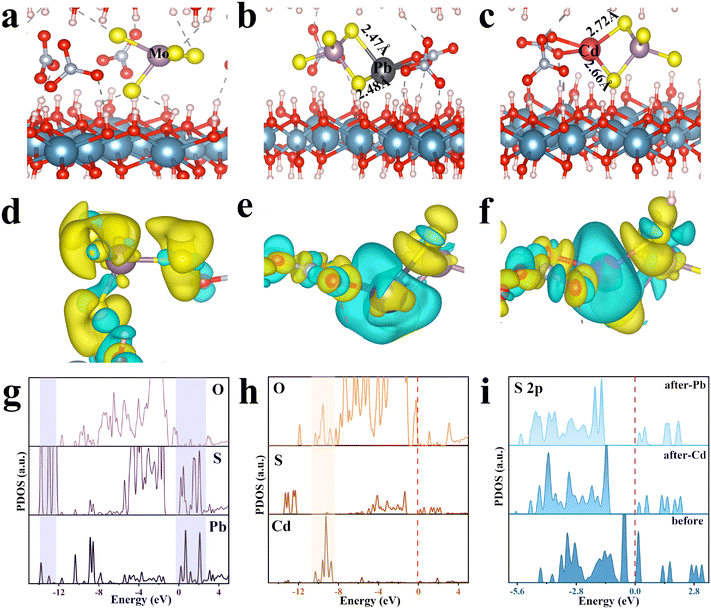
DFT calculations were employed to further validate the adsorption mechanisms of LDH/MoS4 toward Pb(II) and Cd(II), especially the stereochemical structural nature, and the ion–electron interaction was described using the PAW and GGA method. The structures of LDH/MoS4, LDH/MoS4–Pb and LDH/MoS4–Cd were optimized (Fig. 7a–c), and the adsorption energy was obtained (Table S6†). Pb(II) and Cd(II) were found to enter the interlayer in a spontaneous reaction according to the binding energy (Eb) for LDH/MoS4 toward Pb(II) and Cd(II) (Table S6†). As shown in Fig. 7b and c, the Pb–S bond lengths in the LDH/MoS4–Pb conformation were 2.47 Å and 2.48 Å, while the Cd–S bond lengths of LDH/MoS4–Cd were 2.72 Å and 2.66 Å, meaning that S bonds more readily form covalent bonds with Pb in comparison with Cd.57 Furthermore, as seen from the differential charge density distribution mapping in Fig. 7d–f, when [MoS4]2− was introduced in the interlayers of CaAl-LDHs, S and its surroundings interacted to gain electrons. Upon adsorption, Pb(II) was trapped by [MoS4]2− and had a stronger ability to lose electrons, showing larger blue regions, while Cd(II), which is less capable of losing electrons, was trapped, displaying relatively small blue regions.The projected energy density of states (PDOS) of Pb, Cd, S and O after metal adsorption was compared (Fig. 7g and h). Obviously, Pb overlapped more with the S orbital (Fig. 7g), while Cd overlapped more with the O orbital (Fig. 7h). There is a clear covalent feature in the adsorption model, indicating a better formation energy and more charge transfer during adsorption, which just verifies the previous experimental results. To further understand the interaction of S with heavy metals, the PDOS calculations of both S 2s (Fig. S6†) and S 2p (Fig. 7i) were conducted. As seen, both the PDOS peaks of S 2s and S 2p shifted to the lesser energy after adsorption of Pb(II) and Cd(II), demonstrating that a covalent bonding reaction occurred.58 Notably, the PDOS peak after adsorption of Pb(II) shifted more to the lesser energy than that after adsorption of Cd(II), suggesting that the intensity of the bonding reaction between S and Pb was greater than that with Cd,59 which also verified the previous experimental results.
4. Conclusion
In summary, [MoS4]2− clusters were successfully inserted into the LDH interlayer using a facile coprecipitation and ion exchange method which significantly improved the removal of Pb(II) and Cd(II). The enhancement of Pb(II) and Cd(II) removal efficiency could be due to the following: (1) the introduction of [MoS4]2− nanoclusters expanded the substrate spacing and increased the specific surface area of the LDHs, enabling more Pb(II) and Cd(II) to enter the interlayer; (2) Pb(II) and Cd(II) can form strong M–S covalent bonds with the intercalated sulfides which have high selectivity toward Pb(II) and Cd(II); and (3) Cd(II) can easily undergo lattice substitution with Ca(II) in the LDHs, thus achieving Cd immobilization. The results of a series of characterizations and DFT calculations validated the hypothesis that the main adsorption mechanisms are different for Pb(II) and Cd(II). In the case of Pb(II) adsorption, it is mainly through Pb–S bonding that generates precipitation, whereas for Cd(II) immobilization, it is primarily through lattice substitution of Ca with Cd. Overall, our research suggested that LDH/MoS4 has potential for industrial applications in the decontamination of heavy metals at the concentrations of practical wastewater and soils.Author contributions
Fangshu He: conceptualization, data curation, writing – original draft. Zhihui Yang: project administration, resources, supervision. Feiping Zhao: methodology, project administration, supervision, writing – review & editing. Eveliina Repo: writing – review & editing. Weichun Yang: writing – review & editing. Qi Liao: methodology. Mengying Si: methodology. Bin Zou: project administration. Zhang Lin: project administration, resources, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2020YFC1808002, 2019YFA0210403, 2021YFC1809203), the National Natural Science Foundation of China (U20A20267, 52104406), the Natural Science Foundation of Hunan Province (2022JJ20074), and the Academy of Finland (330076). We also thank anonymous reviewers for their constructive comments and suggestions.References
- H. Xiang, X. Min, C.-J. Tang, M. Sillanpää and F. Zhao, Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review, J. Water Process. Eng., 2022, 49, 103023 CrossRef.
- D. Wang, E. Repo, F. He, X. Zhang, H. Xiang, W. Yang, X. Min and F. Zhao, Dual functional sites strategies toward enhanced heavy metal remediation: Interlayer expanded Mg-Al layered double hydroxide by intercalation with L-cysteine, J. Hazard. Mater., 2022, 439, 129693 CrossRef CAS PubMed.
- M. G. K. Houessionon, E. D. Ouendo, C. Bouland, S. A. Takyi, N. M. Kedote, B. Fayomi, J. N. Fobil and N. Basu, Environmental Heavy Metal Contamination from Electronic Waste (E-Waste) Recycling Activities Worldwide: A Systematic Review from 2005 to 2017, Int. J. Environ. Res. Public Health, 2021, 18, 3517 CrossRef CAS.
- Z. Cheng, J. Yang, L. Li, Y. Chen and X. Wang, Flocculation inspired combination of layered double hydroxides and fulvic acid to form a novel composite adsorbent for the simultaneous adsorption of anionic dye and heavy metals, J. Colloid Interface Sci., 2022, 618, 386–398 CrossRef CAS PubMed.
- X. Chen, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Modified-MOF-808-Loaded Polyacrylonitrile Membrane for Highly Efficient, Simultaneous Emulsion Separation and Heavy Metal Ion Removal, ACS Appl. Mater. Interfaces, 2020, 12, 39227–39235 CrossRef CAS.
- Y. Sun, S. Zhou, S.-Y. Pan, S. Zhu, Y. Yu and H. Zheng, Performance evaluation and optimization of flocculation process for removing heavy metal, Chem. Eng. J., 2020, 385, 123911 CrossRef CAS.
- Z. Yang, H. Gong, F. He, E. Repo, W. Yang, Q. Liao and F. Zhao, Iron-doped hydroxyapatite for the simultaneous remediation of lead-, cadmium- and arsenic-co-contaminated soil, Environ. Pollut., 2022, 312, 119953 CrossRef CAS.
- T. Wang, C. Li, C. Wang and H. Wang, Biochar/MnAl-LDH composites for Cu (II) removal from aqueous solution, Colloids Surf., A, 2018, 538, 443–450 CrossRef CAS.
- C. Zhou, X. Wang, X. Song, Y. Wang, D. Fang, S. Ge and R. Zhang, Insights into dynamic adsorption of lead by nano-hydroxyapatite prepared with two-stage ultrasound, Chemosphere, 2020, 253, 126661 CrossRef CAS PubMed.
- F. Chai, R. Zhang, X. Min, Z. Yang, L. Chai and F. Zhao, Highly efficient removal of arsenic (III/V) from groundwater using nZVI functionalized cellulose nanocrystals fabricated via a bioinspired strategy, Sci. Total Environ., 2022, 842, 156937 CrossRef CAS PubMed.
- S. Gu, X. Kang, L. Wang, E. Lichtfouse and C. Wang, Clay mineral adsorbents for heavy metal removal from wastewater: a review, Environ. Chem. Lett., 2018, 17, 629–654 CrossRef.
- M. Zubair, M. Daud, G. McKay, F. Shehzad and M. A. Al-Harthi, Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation, Appl. Clay Sci., 2017, 143, 279–292 CrossRef CAS.
- L. Chen, H. Xu, J. Xie, X. Liu, Y. Yuan, P. Liu, Z. Qu and N. Yan, [SnS4]4- clusters modified MgAl-LDH composites for mercury ions removal from acid wastewater, Environ. Pollut., 2019, 247, 146–154 CrossRef CAS PubMed.
- R. Zhang, Y. Ai and Z. Lu, Application of Multifunctional Layered Double Hydroxides for Removing Environmental Pollutants: Recent Experimental and Theoretical Progress, J. Environ. Chem. Eng., 2020, 8, 103908 CrossRef CAS.
- K. H. Goh, T. T. Lim and Z. Dong, Application of layered double hydroxides for removal of oxyanions: a review, Water Res., 2008, 42, 1343–1368 CrossRef CAS PubMed.
- M. Laipan, J. Yu, R. Zhu, J. Zhu, A. T. Smith, H. He, D. O'Hare and L. Sun, Functionalized layered double hydroxides for innovative applications, Mater. Horiz., 2020, 7, 715–745 RSC.
- D. Chaillot, S. Bennici and J. Brendle, Layered double hydroxides and LDH-derived materials in chosen environmental applications: a review, Environ. Sci. Pollut. Res., 2021, 28, 24375–24405 CrossRef CAS.
- H. Asiabi, Y. Yamini, M. Shamsayei and E. Tahmasebi, Highly selective and efficient removal and extraction of heavy metals by layered double hydroxides intercalated with the diphenylamine-4-sulfonate: A comparative study, Chem. Eng. J., 2017, 323, 212–223 CrossRef CAS.
- C. Lu, W. Qian, M. Mallet, C. Ruby and H. C. B. Hansen, Tuning the stability and phosphate sorption of novel MnII/IVFeII/III layered double hydroxides, Chem. Eng. J., 2022, 429, 132177 CrossRef CAS.
- S. Huang, T. Ouyang, J. Chen, Z. Wang, S. Liao, X. Li and Z. Q. Liu, Synthesis of nickel-iron layered double hydroxide via topochemical approach: Enhanced surface charge density for rapid hexavalent chromium removal, J. Colloid Interface Sci., 2022, 605, 602–612 CrossRef CAS PubMed.
- S. Zhu, M. Asim Khan, F. Wang, Z. Bano and M. Xia, Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: A combined experimental and DFT study, Chem. Eng. J., 2020, 392, 123711 CrossRef CAS.
- F. Zhao, S. Chen, H. Xiang, T. Gao, D. Wang, D. Wei, M. Sillanpää, Y. Ke and C.-J. Tang, Selectively capacitive recovery of rare earth elements from aqueous solution onto Lewis base sites of pyrrolic-N doped activated carbon electrodes, Carbon, 2022, 197, 282–291 CrossRef CAS.
- A. Jawad, Z. Liao, Z. Zhou, A. Khan, T. Wang, J. Ifthikar, A. Shahzad, Z. Chen and Z. Chen, Fe-MoS4: An Effective and Stable LDH-Based Adsorbent for Selective Removal of Heavy Metals, ACS Appl. Mater. Interfaces, 2017, 9, 28451–28463 CrossRef CAS PubMed.
- L. Xie, Z. Yu, S. M. Islam, K. Shi, Y. Cheng, M. Yuan, J. Zhao, G. Sun, H. Li, S. Ma and M. G. Kanatzidis, Remarkable Acid Stability of Polypyrrole-MoS 4 : A Highly Selective and Efficient Scavenger of Heavy Metals Over a Wide pH Range, Adv. Funct. Mater., 2018, 28, 1800502 CrossRef.
- Y. Oh, S. Bag, C. D. Malliakas and M. G. Kanatzidis, Selective Surfaces: High-Surface-Area Zinc Tin Sulfide Chalcogels, Chem. Mater., 2011, 23, 2447–2456 CrossRef CAS.
- L. Ma, Q. Wang, S. M. Islam, Y. Liu, S. Ma and M. G. Kanatzidis, Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS4(2-) Ion, J. Am. Chem. Soc., 2016, 138, 2858–2866 CrossRef CAS PubMed.
- A. Jawad, L. Peng, Z. Liao, Z. Zhou, A. Shahzad, J. Ifthikar, M. Zhao, Z. Chen and Z. Chen, Selective removal of heavy metals by hydrotalcites as adsorbents in diverse wastewater: Different intercalated anions with different mechanisms, J. Cleaner Prod., 2019, 211, 1112–1126 CrossRef CAS.
- J. Ali, H. Wang, J. Ifthikar, A. Khan, T. Wang, K. Zhan, A. Shahzad, Z. Chen and Z. Chen, Efficient, stable and selective adsorption of heavy metals by thio-functionalized layered double hydroxide in diverse types of water, Chem. Eng. J., 2018, 332, 387–397 CrossRef CAS.
- L. Ma, S. M. Islam, C. Xiao, J. Zhao, H. Liu, M. Yuan, G. Sun, H. Li, S. Ma and M. G. Kanatzidis, Rapid Simultaneous Removal of Toxic Anions [HSeO3](−), [SeO3](2-), and [SeO4](2-), and Metals Hg(2+), Cu(2+), and Cd(2+) by MoS4(2-) Intercalated Layered Double Hydroxide, J. Am. Chem. Soc., 2017, 139, 12745–12757 CrossRef CAS PubMed.
- A. F. D. Silva, J. L. D. S. Duarte and L. Meili, Different routes for MgFe/LDH synthesis and application to remove pollutants of emerging concern, Sep. Purif. Technol., 2021, 264, 118353 CrossRef CAS.
- J. Zhang, Z. Lin, Y. Lan, G. Ren, D. Chen, F. Huang and M. Hong, A multistep oriented attachment kinetics: coarsening of ZnS nanoparticle in concentrated NaOH, J. Am. Chem. Soc., 2006, 128(39), 12981–12987 CrossRef CAS PubMed.
- P. E. Blochl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter, 1994, 50, 17953–17979 CrossRef PubMed.
- S. Jana, K. Sharma and P. Samal, Assessing the performance of the recent meta-GGA density functionals for describing the lattice constants, bulk moduli, and cohesive energies of alkali, alkaline-earth, and transition metals, J. Chem. Phys., 2018, 149, 164703 CrossRef.
- R. Bi, D. Yin, B. Lei, F. Chen, R. Zhang and W. Li, Mercaptocarboxylic acid intercalated MgAl layered double hydroxide adsorbents for removal of heavy metal ions and recycling of spent adsorbents for photocatalytic degradation of organic dyes, Sep. Purif. Technol., 2022, 289, 120741 CrossRef CAS.
- F. Chengqian, D. Yimin, C. Ling, W. Zhiheng, L. Qi, L. Yaqi, C. Ling, L. Bo, Z. Yue-Fei, L. Yan and W. Li, One-step coprecipitation synthesis of Cl− intercalated Fe3O4@SiO2 @MgAl LDH nanocomposites with excellent adsorption performance toward three dyes, Sep. Purif. Technol., 2022, 295, 121227 CrossRef.
- B. Ma, A. Fernandez-Martinez, S. Grangeon, C. Tournassat, N. Findling, S. Carrero, D. Tisserand, S. Bureau, E. Elkaim, C. Marini, G. Aquilanti, A. Koishi, N. C. M. Marty and L. Charlet, Selenite Uptake by Ca-Al LDH: A Description of Intercalated Anion Coordination Geometries, Environ. Sci. Technol., 2018, 52, 1624–1632 CrossRef CAS PubMed.
- C. Sun, G. Song, L. Chen, X. Ren and C. Chen, Three dimensional flower-like magnetic polyethyleneimine@MoS2 composites for highly efficient removal of Cr(VI) and Pb(II) ions, J. Colloid Interface Sci., 2020, 580, 550–560 CrossRef CAS PubMed.
- F. Zhao, Y. Liu, S. B. Hammouda, B. Doshi, N. Guijarro, X. Min, C.-J. Tang, M. Sillanpää, K. Sivula and S. Wang, MIL-101(Fe)/g-C3N4 for enhanced visible-light-driven photocatalysis toward simultaneous reduction of Cr(VI) and oxidation of bisphenol A in aqueous media, Appl. Catal., A, 2020, 272, 119033 CrossRef CAS.
- J. Wang and X. Guo, Adsorption isotherm models: Classification, physical meaning, application and solving method, Chemosphere, 2020, 258, 127279 CrossRef CAS PubMed.
- F. Zhao, Z. Yang, Z. Wei, R. Spinney, M. Sillanpää, J. Tang, M. Tam and R. Xiao, Polyethylenimine-modified chitosan materials for the recovery of La(III) from leachates of bauxite residue, Chem. Eng. J., 2020, 388, 124307 CrossRef CAS.
- M. A. Al-Ghouti and D. A. Da'ana, Guidelines for the use and interpretation of adsorption isotherm models: A review, J. Hazard. Mater., 2020, 393, 122383 CrossRef CAS PubMed.
- J. Zhang, Physical insights into kinetic models of adsorption, Sep. Purif. Technol., 2019, 229, 115832 CrossRef CAS.
- J. Wang and X. Guo, Adsorption kinetic models: Physical meanings, applications, and solving methods, J. Hazard. Mater., 2020, 390, 122156 CrossRef CAS.
- X. Li, X. Min, X. Hu, Z. Jiang, C. Li, W. Yang and F. Zhao, In-situ synthesis of highly dispersed Cu-CuxO nanoparticles on porous carbon for the enhanced persulfate activation for phenol degradation, Sep. Purif. Technol., 2021, 276, 119260 CrossRef CAS.
- S. Singh, D. Kapoor, S. Khasnabis, J. Singh and P. C. Ramamurthy, Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials, Environ. Chem. Lett., 2021, 19, 2351–2381 CrossRef CAS.
- L. M. S. Silva, M. J. Muñoz-Peña, J. R. Domínguez-Vargas, T. González and E. M. Cuerda-Correa, Kinetic and equilibrium adsorption parameters estimation based on a heterogeneous intraparticle diffusion model, Surf. Interfaces, 2021, 22, 100791 CrossRef CAS.
- M. B. Asif, H. Kang and Z. Zhang, Gravity-driven layered double hydroxide nanosheet membrane activated peroxymonosulfate system for micropollutant degradation, J. Hazard. Mater., 2022, 425, 127988 CrossRef CAS PubMed.
- X. Wang, H. Li, C. Shan and B. Pan, Construction of model platforms to probe the confinement effect of nanocomposite-enabled water treatment, Chem. Eng. J. Adv., 2022, 9, 100229 CrossRef CAS.
- J. Zhao, L. Zhang, S. Zhang, W. Yuan, X. Fang, Q. Yu and X. Qiu, Remediation of chromium-contaminated soil using calcined layered double hydroxides containing different divalent metals: Temperatures and mechanism, Chem. Eng. J., 2021, 425, 131405 CrossRef CAS.
- S. Zhang, J. Chen, J. Yu, Q. Yu and X. Qiu, Remediation of Cd-contaminated soil through different layered double hydroxides: The weakness of delamination and mechanism, J. Environ. Chem. Eng., 2022, 10, 107815 CrossRef CAS.
- X. Wang, Y. Tuo, Y. Zhou, D. Wang, S. Wang and J. Zhang, Ta-doping triggered electronic structural engineering and strain effect in NiFe LDH for enhanced water oxidation, Chem. Eng. J., 2021, 403, 126297 CrossRef CAS.
- J. Wang, W. Zhang, X. Yue, Q. Yang, F. Liu, Y. Wang, D. Zhang, Z. Li and J. Wang, One-pot synthesis of multifunctional magnetic ferrite–MoS2–carbon dot nanohybrid adsorbent for efficient Pb(ii) removal, J. Mater. Chem. A, 2016, 4, 3893–3900 RSC.
- N. Kumar, E. Fosso-Kankeu and S. S. Ray, Achieving Controllable MoS2 Nanostructures with Increased Interlayer Spacing for Efficient Removal of Pb(II) from Aquatic Systems, ACS Appl. Mater. Interfaces, 2019, 11, 19141–19155 CrossRef CAS.
- W. Liu, F. Huang, Y. Liao, J. Zhang, G. Ren, Z. Zhuang, J. Zhen, Z. Lin and C. Wang, Treatment of CrVI-Containing Mg(OH)2 Nanowaste, Angew. Chem., Int. Ed., 2008, 47, 5619–5622 CrossRef CAS PubMed.
- Y. Zhang, J. Qu, Y. Yuan, H. Song, Y. Liu, S. Wang, Y. Tao, Y. Zhao and Z. Li, Simultaneous scavenging of Cd(II) and Pb(II) from water by sulfide-modified magnetic pinecone-derived hydrochar, J. Cleaner Prod., 2022, 341, 130758 CrossRef CAS.
- Q. Li, L. Wang, R. Xu, Y. Yang, H. Yin, S. Jin and T. Jiang, Potentiality of phosphorus-accumulating organisms biomasses in biosorption of Cd(II), Pb(II), Cu(II) and Zn(II) from aqueous solutions: Behaviors and mechanisms, Chemosphere, 2022, 303, 135095 CrossRef CAS PubMed.
- D. You, H. Shi, L. Yang, P. Shao, K. Yin, H. Wang, S. Luo and X. Luo, Tuning the Sb(V) adsorption performance of La-MOFs via ligand engineering effect: Combined experiments with theoretical calculations, Chem. Eng. J., 2022, 435, 134874 CrossRef CAS.
- Z. Feng, N. Chen, T. Liu and C. Feng, KHCO3 activated biochar supporting MgO for Pb(II) and Cd(II) adsorption from water: Experimental study and DFT calculation analysis, J. Hazard. Mater., 2022, 426, 128059 CrossRef CAS.
- F. Guo, D. Li, J. B. Fein, J. Xu, Y. Wang, Q. Huang and X. Rong, Roles of hydrogen bond and ion bridge in adsorption of two bisphenols onto montmorillonite: an experimental and DFT study, Appl. Clay Sci., 2022, 217, 106406 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2en00934j |
| This journal is © The Royal Society of Chemistry 2023 |