Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Poly-phenylene jacketed tailor-made dendritic phenylazomethine ligand for nanoparticle synthesis†
Ken
Albrecht‡§
 *abc,
Maki
Taguchi‡
b,
Takamasa
Tsukamoto§
ab,
Tatsuya
Moriai
a,
Nozomi
Yoshida
a and
Kimihisa
Yamamoto
*abc,
Maki
Taguchi‡
b,
Takamasa
Tsukamoto§
ab,
Tatsuya
Moriai
a,
Nozomi
Yoshida
a and
Kimihisa
Yamamoto
 *ab
*ab
aLaboratory for Chemistry and Life Science (CLS), Institute of Innovative Research (IIR), Tokyo Institute of Technology, Yokohama 226-8503, Japan. E-mail: yamamoto@res.titech.ac.jp
bJST-ERATO, Yamamoto Atom Hybrid Project, Institute of Innovative Research (IIR), Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan
cInstitute for Materials Chemistry, Engineering Kyushu University, 6-1 Kasuga-Koen Kasuga-shi, 816-8580, Fukuoka, Japan. E-mail: albrecht@cm.kyushu-u.ac.jp
First published on 18th March 2022
Abstract
Synthesizing metal clusters with a specific number of atoms on a preparative scale for studying advanced properties is still a challenge. The dendrimer templated method is powerful for synthesizing size or atomicity controlled nanoparticles. However, not all atomicity is accessible with conventional dendrimers. A new tailor-made phenylazomethine dendrimer (DPA) with a limited number of coordination sites (n = 16) and a non-coordinating large poly-phenylene shell was designed to tackle this problem. The asymmetric dendron and adamantane core four substituted dendrimer (PPDPA16) were successfully synthesized. The coordination behavior confirmed the accumulation of 16 metal Lewis acids (RhCl3, RuCl3, and SnBr2) to PPDPA16. After the reduction of the complex, low valent metal nanoparticles with controlled size were obtained. The tailor-made dendrimer is a promising approach to synthesize a variety of metal clusters with desired atomicity.
Introduction
Metal nanoparticles are widely used in several applications and their property changes as a function of their size.1,2 However, in the sub-nanometer region, controlling the structure and atomicity is essential to control the property because the surface atoms will be dominant, and the particle will have a molecular electronic structure instead of a band-like electronic structure.3,4 Atomicity-controlled synthesis of nanoparticles (clusters) can be achieved with the gas-phase synthesis in combination with mass sorting techniques.5–8 This allowed the spectroscopic study of several metals to metal oxides nanoparticles and opened the door of cluster science. However, the limitation is the low yield and slow deposition rate of the clusters, leading to difficulties in measuring advanced properties such as catalytic properties and handling them practically. A liquid-phase synthesis is a promising approach for the preparative scale production of atomicity-controlled clusters suitable for observing advanced properties. Some so-called “magic-number” clusters can be obtained as a thermodynamically stable product on a relatively large scale.9,10 However, these clusters are usually covered with ligands, and not all atomicity is accessible. The ligand usually suppresses the catalytic reactivity, and the ligand structure dominates the electronic property. Therefore, a new method is eagerly anticipated to synthesize unprotected or weakly protected and atomicity-controlled clusters on a preparative scale for studying the bare property of the clusters.Dendrimers11–16 that consist of multiple ligands can provide storage of a limited number of metal salts that lead to nanoparticle formation with a small size distribution.17–21 Phenylazomethine dendrimer (DPA)22–28 is a unique scaffold to assemble a specific number of Lewis acids and form weakly stabilized and kinetically trap nanoparticles to the internal space.29 DPA has a π-conjugated rigid backbone and a dense shell (sparse core).30 This character is suitable for trapping nanoparticles inside the dendrimer but keeping accessible space around the nanoparticle. As a result, the reactant can access the nanoparticle surface, and reactivity of DPA encapsulated nanoparticles will be maintained.31,32 However, due to the branched molecular structure and the stepwise radial complexation behavior, the number of Lewis acids that can be finely assembled into DPAs are usually limited to n, 3n, 7n, and 15n (n = core substitution number, see Fig.S1† for detail). Adopting an asymmetric core33 or inversion of the coordination sequence34 can allow other Lewis acid assembly numbers. However, this approach is far from covering all numbers in the range of 1 to 30 atoms, limiting the access to series of nanoparticles with each atomicity.
A straightforward solution to create precursors for nanoparticles with specific atomicity is to prepare a polynuclear complex35,36 consisting of a ligand with a specific number of coordination sites. DPA with a tailor-made number of azomethine units matches this criterion; however, low generation asymmetric DPAs are required to achieve low atomicity. This will cause a challenge in synthesis and the lack of “shell effect” necessary to preserve the formed nanoparticle inside the dendrimer. Here we will propose the concept of a new tailor-made DPA with regulated coordination sites and a large non-coordinating shell (polyphenylene). The synthesis of asymmetric dendron and dendrimer with exactly 16 coordination sites and the capability as nanoparticle template will be demonstrated. This will be a versatile approach to have dendritic ligands with regulated coordination sites and sufficient shell effect for nanoparticle synthesis.
Results and discussion
The new DPA was designed to fulfill the following requirements; (1) having a regulated number of azomethine units, and (2) having a sufficient shell to kinetically trap the formed particles. Asymmetric phenylazomethine dendrimers with various phenylazomethine units can form a polynuclear complex with a specific number of Lewis acids. However, with this simple structure, the nanoparticle created in the dendrimers by reduction or oxidation of assembled Lewis acids will rapidly leach out the dendrimer scaffold. To provide a shell around the phenylazomethine units, a non-coordinating dendritic structure that is stable under the convergent synthesis of DPA, i.e., the dehydration reaction with TiCl4 and oxidation with KMnO4, is required.37 Polyphenylene (PP) dendrimer is a rigid π-conjugated dendrimer consisting of benzene rings38 and has sufficient stability under the above reaction conditions. Phenylenevinylene dendrimers39,40 have a similar structure and were also considered as a candidate to cover DPA, but it was not stable under the KMnO4 oxidation process. Therefore, PP dendrimer was chosen as a shell to cover DPA, and the dendrimer was designed to have in total 4 times (DPA + PP) branching to form a jacket (Chart 1). The new dendrimer design concept using dendrons with asymmetric dendrons can generally be applied to synthesize dendrimers with various phenylazomethine units through controlling the combination and substitution number of dendrons.36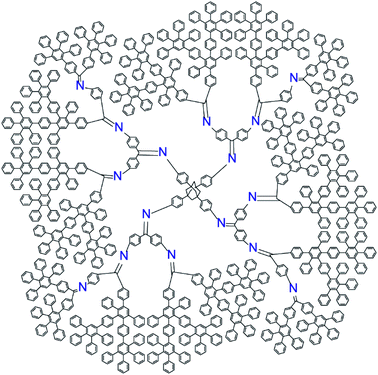 | ||
| Chart 1 Structure of phenylazomethine dendrimer with exactly 16 coordination sites and polyphenylene jacket (PPDPA16). | ||
The new dendrimer with exactly 16 azomethine coordination sites with a PP shell was synthesized with the combination of divergent and convergent methods. First, the 1st and 2nd generation PP dendron was attached to a benzophenone unit with the divergent method.41 Next, the azomethine units were synthesized with the convergent method that the dehydration reaction between benzophenone and aniline is the key. Finally, the asymmetric dendron was reacted with an adamantane core to form the dendrimer with 16 coordination sites(Scheme S1–5†). Note that the tetraphenylmethane30 was too small to bind four dendrons due to the large steric hindrance of the dendrons and the dehydration reaction stopped after the 3-amine groups reacted. All compounds were characterized with 1H and 13C NMR, MALDI-TOF-MS, and elemental analysis. The solubility of the dendrimer in THF was low compared to conventional DPA but soluble in chloroform reflecting the character of the PP shell. The asymmetric dendron with 4 azomethine units requires longest synthetic route compared to other symmetric dendrons (3 and 7) that are required to synthesize dendrimers with various phenylazomethine units.36 With the success in the synthesis of PPDPA16, we have demonstrated that the design concept of coordination site regulated DPA can be realized.
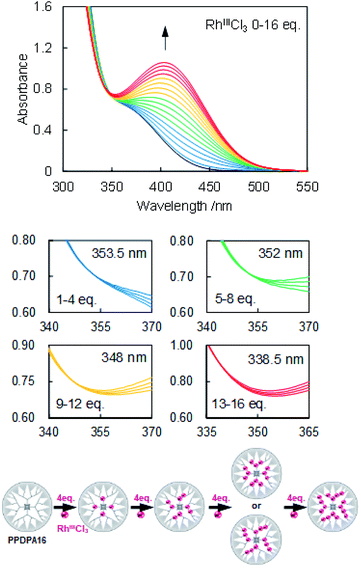
The coordination behavior of the dendrimer with RhCl3, RuCl3, and SnBr2 was determined by UV-vis titration (Fig. 1 and S3–5†). Upon adding RhCl3 to the dendrimer solution (5 μM, dichloromethane), typical change of the UV-vis spectra according to the complexation of Lewis acids to phenylazomethine, i.e., the increase of the absorption at 400 nm and decrease at shorter wavelength was observed (Fig. S5 and 6†).24 The careful evaluation revealed the existence of 4 isosbestic points during the addition of 16 eq. of RhCl3. The spectra change has continued after the addition of more than 16 eq. without an isosbestic point. This is attributed to other reaction that is not coordination to phenylazomethine unit. The 4 isosbestic points until addition of 16 eq. indicate that RhCl3 coordinates to PPDPA16 in a stepwise manner. The isosbestic point changes every 4 eq. of RuCl3 and by comparison with previous reports, it is attributed to the stepwise complexation from the inner-layer to the outer-layer units.24 The sequence cannot be exactly determined, but possibly can be the sequence illustrated in Fig. 1 down. Considering that azomethine unit where another azomethine unit is bound to the outside has stronger basicity due to the electron donation.36 In the case of RuCl3 and SnBr2, the UV-vis spectra of dendrimer solution also showed a similar trend (Fig. S3 and 4†). Most importantly, these data mean that PPDPA16 has the capability to exactly collect 16 metal atoms into the dendrimer scaffold.
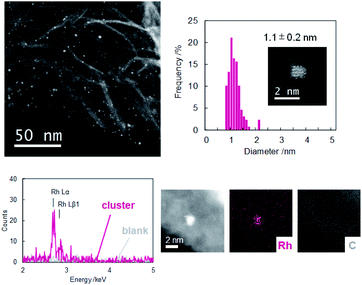
Dendrimer templated Rh nanoparticles42,43 were synthesized by reducing PPDPA16-(RhCl3)16 complex. To the solution of the complex, NaBH4 methanol solution was added under vigorous stirring. The color change corresponding to decomplexation was observed, and immediately GNP (graphene nanopowder) dispersed in dichloromethane (1 mg/2 mL) was added to support dendrimer encapsulated nanoparticles. The dispersion was cast on a TEM grid, and HAADF-STEM images were obtained (Fig. 2). The image of Rh clusters with size of 1.1 ± 0.2 nm were obtained. The size corresponds well with a model of Rh16 cluster within the error range, and the EDX analysis has confirmed that the particle consists of Rh atoms. During obtaining the high-magnification image, the disassembly of the particle was observed due to the high energy of the electron beam. The image after disassembly of the one particle clearly showed that this particle consists of 16 bright spots (Fig. S11†). These results do not secure that all particles formed in PPDPA16 are exactly Rh16 particles, but most likely, the majority is Rh16 cluster weakly stabilized with the DPA and PP scaffold31 as expected from the dendrimer design.
The entire coordination and reduction process of RhCl3 in PPDPA16 was also investigated with XPS (Fig. S9†). RhCl3 showed the peak at a binding energy of 311.5 eV that is assigned to the 3d5/2 orbital. Coordination of 16RhCl3 to imine sites has shifted the binding energy and broadened the signals to the lower region (310.2 eV) due to the electron donation from the nitrogen lone pair. The following reduction shifted the binding energy to 307.5 eV, corresponding to low valent Rh metal (it is assumed that it mainly consists of 0 valent with some oxidized state).26 Further investigation and fitting of the XPS spectra of RhCl3 coordinating to the dendrimer revealed that the peak consists of three components with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 strength ratio (Fig. S9†). Considering the sequential coordination behavior and the difference in the basicity of each imine site, it is assumed that this reflects the number of RhCl3 that is coordinating to different imine sites. Namely, the peak at 308.6 eV with the lowest binding energy is the Rh atom with the most strong electron donated RhCl3 that corresponds to four RhCl3 coordinating to the innermost imine sites of PPDPA16. The peak at the highest binding energy (311.4 eV) is assigned to four RhCl3 coordinating to the weakest (low basicity) imine site. The intermediate peak at 310.2 eV is attributed to eight (four + four) RhCl3 coordinates second and third to PPDPA16. Overall, the XPS analysis confirmed the sequential coordination of RhCl3 to PPDPA16 and the reduction to metallic Rh particles.
1 strength ratio (Fig. S9†). Considering the sequential coordination behavior and the difference in the basicity of each imine site, it is assumed that this reflects the number of RhCl3 that is coordinating to different imine sites. Namely, the peak at 308.6 eV with the lowest binding energy is the Rh atom with the most strong electron donated RhCl3 that corresponds to four RhCl3 coordinating to the innermost imine sites of PPDPA16. The peak at the highest binding energy (311.4 eV) is assigned to four RhCl3 coordinating to the weakest (low basicity) imine site. The intermediate peak at 310.2 eV is attributed to eight (four + four) RhCl3 coordinates second and third to PPDPA16. Overall, the XPS analysis confirmed the sequential coordination of RhCl3 to PPDPA16 and the reduction to metallic Rh particles.
The formation of Ru nanoparticle44,45 through the reduction of PPDA16-(RuCl3)16 complex was also performed similarly to Rh. The coordination of RuCl3 to PPDPA16 and reduction to low state valent was confirmed by XPS measurements(Fig. S10†). The binding energy of RuCl3 (Ru 3p3/2) was 463.9 eV and shifted to lower binding energy (462.1 eV) due to the electron donation from the imine nitrogen. The reduction of the complex further shifted the binding energy to 461.2 eV, indicating the reduction to a low valent state that mainly consists of 0 valent.26 The obtained particle size was determined from STEM images to be 1.0 ± 0.1 nm (Fig. S12†). This matches the size of Ru16 cluster within experimental error. These observations indicate that PPDPA16 has the capability to collect 16 Lewis acids through coordination to imine nitrogen, and reduction of the complex will give metal particles within a specific atomicity range.
To confirm the effectiveness of the PP jacket to inhibit aggregation of the nanoparticles, a reference experiment using DPA with 12 coordination sites without a PP jacket (DPA12, Fig. S2†) was performed. The UV-vis titration of DPA12 with RhCl3 and RuCl3 indicates that DPA12 has the capability to coordinate exactly 12 Lewis acids (Fig. S7 and 8†). These complexes were reduced, supported on GNP, and STEM images were obtained. The observed size of the particles was 1.4 ± 0.6 nm and 1.0 ± 0.2 nm for Rh and Ru particles, respectively (Fig. S13 and 14†). These values are indicating that the particles are undergoing aggregation. Furthermore, the same experiments were performed without any dendrimer. This experiment resulted in a large aggregate formation (Fig. S15†). This indicates that in the absence of the PP jacket, reduced metal salts will undergo aggregation and the existence of the large PP jacket is the key to form clusters with a specific atomicity range.
Conclusions
In conclusion, phenylazomethine dendrimer with exactly 16 coordination sites covered with a polyphenylene dendrimer jacket was synthesized. This dendrimer (PPDPA16) can collect 16 Lewis acids through coordination to imine sites. Reduction of RhCl3 and RuCl3 complex with NaBH4 resulted in nanoparticles consisting of low valent metals with a narrow distribution. This is only one example of a tailor-made dendrimer with a specific number of coordination sites, but in principle, this strategy will lead to synthesizing a variety of dendrimers with regulated coordination sites and serve as a template for metal clusters with desired atomicity. The new concept of coordination site regulated dendrimer will lead to synthesizing the series of atomicity controlled nanoparticles. Further characterization and study on the electronic structure with EXAFS and catalytic property of series of nanoparticles will be conducted in the future.Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.Author contributions
MT., TT, TM, and NY collected experimental data. KA and KY conceptualized the project and supervised the investigation. KA designed the methodology, and wrote the manuscript with the help of MT and TT.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This study was supported by a grant from JST ERATO Grant Number JPMJER1503, "Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials" from the Ministry of Education, Culture,Sports, Science and Technology of Japan (MEXT), and JSPS KAKENHI grant No. JP21H05023.References
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- T. Takei, T. Akita, I. Nakamura, T. Fujitani, M. Okumura, K. Okazaki, J. Huang, T. Ishida and M. Haruta, Adv. Catal., 2012, 55, 1–126 CAS.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- P. Jena and Q. Sun, Chem. Rev., 2018, 118, 5755–5870 CrossRef CAS PubMed.
- M. Mitsui, S. Nagaoka, T. Matsumoto and A. Nakajima, J. Phys. Chem. B, 2006, 110, 2968–2971 CrossRef CAS PubMed.
- I. Katakuse, T. Ichihara, Y. Fujita, T. Matsuo, T. Sakurai and H. Matsuda, Int. J. Mass Spectrom., 1986, 69, 109–114 CrossRef CAS.
- T. Diederich, T. Döppner, J. Braune, J. Tiggesbäumker and K.-H. Meiwes-Broer, Phys. Rev. Lett., 2001, 86, 4807–4810 CrossRef CAS PubMed.
- A. Herlerta, S. Krückeberga, L. Schweikharda, M. Vogela and C. Walther, J. Electron. Spectrosc., 2000, 106, 179–186 CrossRef.
- W. Kurashige, Y. Niihori, S. Sharma and Y. Negishi, J. Phys. Chem. Lett., 2014, 5, 4134–4142 CrossRef CAS PubMed.
- H. Hirai, S. Ito, S. Takano, K. Koyasu and T. Tsukuda, Chem. Sci., 2020, 11, 12233–12248 RSC.
- D. A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin and J. Roeck, Polym. J., 1985, 17, 117–132 CrossRef CAS.
- D. Astruc, E. Boisselier and C. Ornelas, Chem. Rev., 2010, 110, 1857–1959 CrossRef CAS PubMed.
- M. Fischer and F. Vögtle, Angew. Chem., Int. Ed., 1999, 38, 884–905 CrossRef.
- A. W. Bosman, H. M. Janssen and E. W. Meijer, Chem. Rev., 1999, 99, 1665–1688 CrossRef CAS PubMed.
- G. R. Newkome and C. Shreiner, Chem. Rev., 2010, 110, 6338–6442 CrossRef CAS PubMed.
- S. M. Grayson and J. M. J. Fréchet, Chem. Rev., 2001, 101, 3819–3868 CrossRef CAS PubMed.
- R. M. Crooks, M. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181–190 CrossRef CAS PubMed.
- H. Lang, R. A. May, B. L. Iversen and B. D. Chandler, J. Am. Chem. Soc., 2003, 125, 14832–14836 CrossRef CAS PubMed.
- C. V. Deraedt, R. Ye, W. Ralston, F. D. Toste and G. A. Somorjai, J. Am. Chem. Soc., 2017, 139, 18084–18092 CrossRef CAS PubMed.
- A. K. Diallo, C. Ornelas, L. Salmon, J. R. Aranzaes and D. Astruc, Angew. Chem., Int. Ed., 2007, 46, 8644–8648 CrossRef CAS PubMed.
- K. Yamamoto, T. Imaoka, M. Tanabe and T. Kambe, Chem. Rev., 2020, 120, 1397–1437 CrossRef CAS PubMed.
- K. Yamamoto and T. Imaoka, Acc. Chem. Res., 2014, 47, 1127–1136 CrossRef CAS PubMed.
- K. Yamamoto, M. Higuchi, S. Shiki, M. Tsuruta and H. Chiba, Nature, 2002, 415, 509–511 CrossRef CAS PubMed.
- T. Moriai, T. Tsukamoto, M. Tanabe, T. Kambe and K. Yamamoto, Angew. Chem., Int. Ed., 2020, 59, 23051–23055 CrossRef CAS PubMed.
- K. Albrecht, Y. Hirabayashi, M. Otake, S. Mendori, Y. Tobari, Y. Azuma, Y. Majima and K. Yamamoto, Sci. Adv., 2016, 2, e1601414 CrossRef PubMed.
- M. Huda, K. Minamisawa, T. Tsukamoto, M. Tanabe and K. Yamamoto, Angew. Chem., Int. Ed., 2019, 58, 1002–1006 CrossRef CAS PubMed.
- K. Yamamoto, T. Imaoka, W.-J. Chun, O. Enoki, H. Katoh, M. Takenaga and A. Sonoi, Nat. Chem., 2009, 1, 397–402 CrossRef CAS PubMed.
- N. Satoh, T. Nakashima, K. Kamikura and K. Yamamoto, Nat. Nanotechnol., 2008, 3, 106–111 CrossRef CAS PubMed.
- T. Imaoka, H. Kitazawa, W.-J. Chun, S. Omura, K. Albrecht and K. Yamamoto, J. Am. Chem. Soc., 2013, 135, 13089–13095 CrossRef CAS PubMed.
- O. Enoki, H. Katoh and K. Yamamoto, Org. Lett., 2006, 8, 569–571 CrossRef CAS PubMed.
- T. Imaoka, H. Kitazawa, W. Chun and K. Yamamoto, Angew. Chem., Int. Ed., 2015, 54, 9810–9815 CrossRef CAS PubMed.
- I. Nakamula, Y. Yamanoi, T. Yonezawa, T. Imaoka, K. Yamamoto and H. Nishihara, Chem. Commun., 2008, 5716–5718 RSC.
- H. Kitazawa, K. Albrecht and K. Yamamoto, Chem. Lett., 2012, 41, 828–830 CrossRef CAS.
- K. Albrecht, N. Sakane and K. Yamamoto, Chem. Commun., 2014, 50, 12177–12180 RSC.
- T. Imaoka, Y. Akanuma, N. Haruta, S. Tsuchiya, K. Ishihara, T. Okayasu, W. Chun, M. Takahashi and K. Yamamoto, Nat. Commun., 2017, 8, 688 CrossRef PubMed.
- K. Takada, M. Morita, T. Imaoka, J. Kakinuma, K. Albrecht and K. Yamamoto, Sci. Adv., 2021, 7, eabd9887 CrossRef CAS PubMed.
- K. Takanashi, H. Chiba, M. Higuchi and K. Yamamoto, Org. Lett., 2004, 6, 1709–1712 CrossRef CAS PubMed.
- B. A. G. Hammer and K. Müllen, J. Nanopart. Res., 2018, 20, 262 CrossRef PubMed.
- P. A. Bell and T. M. Chapman, Polym. Prepr., 2007, 48, 520–521 CAS.
- M. Mizusaki, Y. Yamada, S. Obara and K. Tada, Chem. Lett., 2012, 41, 516–517 CrossRef CAS.
- U.-M. Wiesler, A. J. Berresheim, F. Morgenroth, G. Lieser and K. Müllen, Macromolecules, 2001, 34, 187–199 CrossRef CAS.
- H. Guan, J. Lin, B. Qiao, X. Yang, L. Li, S. Miao, J. Liu, A. Wang, X. Wang and T. Zhang, Angew. Chem., Int. Ed., 2016, 55, 2820–2824 CrossRef CAS PubMed.
- C. Lin, G. Wu, H. Li, Y. Geng, G. Xie, J. Yang, B. Liu and J. Jin, Nanoscale, 2017, 9, 1834–1839 RSC.
- J. Ohyama, T. Sato, Y. Yamamoto, S. Arai and A. Satsuma, J. Am. Chem. Soc., 2013, 135, 8016–8021 CrossRef CAS PubMed.
- C. Xu, M. Ming, Q. Wang, C. Yang, G. Fan, Y. Wang, D. Gao, J. Bia and Y. Zhang, J. Mater. Chem. A, 2018, 6, 14380–14386 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc05661a |
| ‡ These authors contributed equally. |
| § Present address: JST-PRESTO, Kawaguchi 332-0012(Japan). |
| This journal is © The Royal Society of Chemistry 2022 |