Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
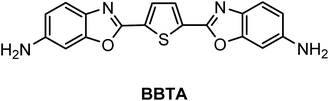
A fluorescent dye with large Stokes shift and high stability: synthesis and application to live cell imaging
Zheng Gaoab,
Yongcao Haoa,
Meiling Zheng a and
Yi Chen
a and
Yi Chen *a
*a
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: yichen@mail.ipc.ac.cn; Fax: +86 10 6487 9375; Tel: +86 10 8254 3595
bUniversity of Chinese Academy of Sciences, Beijing, 100190, China
First published on 25th January 2017
Abstract
A fluorescent dye, 2,5-bis(6-amine-benzoxazol-2-yl)thiophene (BBTA), was synthesized by a two-step reaction from starting material 2,5-bis(benzoxazol-2-yl)thiophene (BBT). BBTA exhibited strong emission and large Stokes shift in solvent, and the largest Stokes shift (Δλ = 186 nm or Δν = 8572 cm−1) was obtained in buffer solution (PBS, pH = 7.2) with 5% (v/v) of polyethylene glycol monomethyl ether (mPEG550, MW = 550) as additive. Application of BBTA to live cell imaging showed that BBTA was clearly expressed in mitochondria with high contrast. Besides, BBTA showed low cytotoxicity and excellent photo-stability.
Introduction
Development of fluorescent organic dyes with large Stokes shift and high photo-stability is essential for biological applications.1–3 Advances in fluorescent organic dyes with large Stokes shift (typically over 80 nm) can minimize cross-talk between the excitation source and the fluorescent emission for cellular imaging with high signal-to-noise ratio.4–6 On the other hand, fluorescent dyes with high photo-stability are beneficial to noninvasive long-term cellular imaging, which is of great significance for investigating biological processes, pathological pathways, and therapeutic effects over long time spans.7–9Typical fluorophore dyes such as fluorescein dyes,10 rhodamine dyes,11 cyanine dyes12–17 nile red dyes,18 and BODIPY dyes19,20 exhibit small Stokes shifts (Δλ ≤ 70 nm) (Table 1),21–23 which can reabsorb emitted photons leading to undesired back-ground interferences. To address this issue, great effects have been dedicated and a number of noted fluorophore dyes with large Stokes shift (Δλ ≥ 80 nm) have been developed.24–28 However, some problems are encountered with them containing complicated structures, multi-steps reaction, and low yields.
| Compound | λexmax (nm) | λemmax (nm) | Δλ (nm) | Δν (cm−1) |
|---|---|---|---|---|
| Fluorescein | 494 | 518 | 24 | 938 |
| 5-Carboxyfluorescein | 484 | 520 | 36 | 1430 |
| Fluorescein isothiocyanate | 494 | 518 | 24 | 938 |
| Rhodamine 123 | 502 | 528 | 26 | 981 |
| Rhodamine 6G | 528 | 552 | 24 | 823 |
| Rhodamine Green™ | 494 | 520 | 26 | 1012 |
| Cy2 | 493 | 506 | 13 | 521 |
| Cy3 (ref. 21) | 550 | 570 | 20 | 638 |
| Cy5 (ref. 22) | 650 | 670 | 20 | 459 |
| Nile red | 580 | 650 | 70 | 1856 |
| BODIPY 493 | 494 | 504 | 10 | 401 |
| BODIPY 505 | 502 | 512 | 10 | 389 |
| Mito-Tracker Green™ | 490 | 516 | 26 | 1028 |
| Mito-Tracker Red™ | 579 | 599 | 20 | 577 |
Photostability is another important parameter in evaluating the practical application of fluorescent dyes for bioimaging, and a high photostability is desirable when a fluorescent dye is used for bioimaging, especially for long-term illumination due to investigating biological processes.29,30 For most small organic fluorescent dyes, poor photostability is their common character. To enhance the photostability of organic fluorophores, nano-technology such as conjugated nanoparticles,31,32 doped in silica nanoparticles33,34 or gold nanoparticles,35,36 and doped to polymer nanoparticles37–39 is employed. In generally, nanoparticles with organic polymers or inorganic matrix can provide the encapsulated or doped dye molecules with better photostability, but some disadvantages such as complicated synthesis, easy micelle dissociation, and limited surface functionalization are contained. Therefore, direct structural modification or molecular engineering of fluorophores continues to be an active research area, and some elegant organic fluorophores with high photostability have been reported.40–44
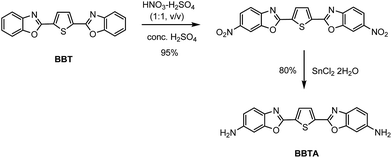
Benzoxazoles are one of the most important class of heterocyclic compounds, not only as key structural units of compounds with interesting biological activities45–47 but also in the field of materials chemistry.48–50 In particular, 2,5-bis(benzoxazol-2-yl)thiophene (BBT) (trade name: Uviter EBF, CAS no. 2866-43-5) (chemical structure see Scheme 2) and its derivatives are well-known fluorescent dyes used as whitening agents51,52 and various optoelectronic applications53–55 due to strong emission and high photo-stability. Herein, a new derivative 2,5-bis(6-amine-benzoxazol-2-yl)thiophene (BBTA)56 (Scheme 1) has been synthesized by a two-step reaction starting from BBT (synthetic route see Scheme 2). BBTA exhibits very large Stokes shift and excellent photo-stability in buffer solution (with 5% of mPEG550 as cosolvent) which is beneficial to biological imaging. Using HeLa cells as prototype, BBTA can be clearly expressed in mitochondria with high contrast.
Experimental
Synthesis
(a) To a solution of 2,5-bis(benzoxazol-2-yl)thiophene (BBT,57 3.18 g, 10 mmol) in conc. H2SO4 (98%, 20 mL) was dropwise added the mixture of HNO3 (65%) and H2SO4 (98%) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 1.3 mL) at 0 °C. The mixture was stirred at 0 °C for 1 h, and then slowly poured into ice water (30 mL). The cold mixture solution was stirred slowly at ambient temperature until yellow precipitate was produced completely. The yellow product was filtered off, washed with water (3 × 30 mL), and dried under vacuum. The crude product (2,5-bis(6-nitrobenzoxazol-2-yl)thiophene) was obtained in yield of 95% and used for the next step without further purification. (b) To the solution of 2,5-bis(6-nitrobenzoxazol-2-yl)thiophene (1.02 g, 2.5 mmol) in EtOH (20 mL) was added SnCl2·2H2O (5.6 g, 25 mmol). The mixture was refluxed till the starting material was disappeared checked by TLC (Rf = 0.51, eluent: PE/EtOAc = 2
1, v/v, 1.3 mL) at 0 °C. The mixture was stirred at 0 °C for 1 h, and then slowly poured into ice water (30 mL). The cold mixture solution was stirred slowly at ambient temperature until yellow precipitate was produced completely. The yellow product was filtered off, washed with water (3 × 30 mL), and dried under vacuum. The crude product (2,5-bis(6-nitrobenzoxazol-2-yl)thiophene) was obtained in yield of 95% and used for the next step without further purification. (b) To the solution of 2,5-bis(6-nitrobenzoxazol-2-yl)thiophene (1.02 g, 2.5 mmol) in EtOH (20 mL) was added SnCl2·2H2O (5.6 g, 25 mmol). The mixture was refluxed till the starting material was disappeared checked by TLC (Rf = 0.51, eluent: PE/EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After cooled to ambient temperature, the mixture solution was then slowly poured into water (200 mL), extracted with CH2Cl2 (5 × 30 mL). The combined organic solution was dried over anhydrous Na2SO4, after evaporation of the solvent, the crude product was purified by flash column chromatography (PE/EtOAc = 1
1, v/v). After cooled to ambient temperature, the mixture solution was then slowly poured into water (200 mL), extracted with CH2Cl2 (5 × 30 mL). The combined organic solution was dried over anhydrous Na2SO4, after evaporation of the solvent, the crude product was purified by flash column chromatography (PE/EtOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, PE: 40–60 °C) to afford BBTA (0.70 g) in yield of 80%, Rf = 0.55 (eluent: EtOAc). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.95 (s, 2H), 7.65 (d, J = 15.6 Hz, 2H), 7.21 (s, 2H), 6.98 (d, J = 8.4 Hz, 2H), 3.76 (brs, 4H). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 156.8, 152.8, 147.9, 132.7, 130.5, 120.9, 115.0, 96.5. HRMS (EI+) calcd for C18H12N4O2S (M+): 348.0681. Found: 348.0682. IR (cm−1): 3406, 2904, 1606, 1534, 1141, 1048.
1, v/v, PE: 40–60 °C) to afford BBTA (0.70 g) in yield of 80%, Rf = 0.55 (eluent: EtOAc). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.95 (s, 2H), 7.65 (d, J = 15.6 Hz, 2H), 7.21 (s, 2H), 6.98 (d, J = 8.4 Hz, 2H), 3.76 (brs, 4H). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 156.8, 152.8, 147.9, 132.7, 130.5, 120.9, 115.0, 96.5. HRMS (EI+) calcd for C18H12N4O2S (M+): 348.0681. Found: 348.0682. IR (cm−1): 3406, 2904, 1606, 1534, 1141, 1048.
Cell culture and fluorescence images
For the fluorescence imaging in live cells, HeLa cells are cultured in culture media Dulbecco's Modified Eagle's Medium (DMEM/F12 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (HyClone) with 10% Fetal Bovine Serum (FBS) and 1% penicillin–streptomycin) at 37 °C under a humidified atmosphere containing 5% CO2 for 24 h. The cells were seeded on a Ø 35 mm glass-bottomed dish (NEST) for live-cell imaging by confocal laser scanning microscopy (CLSM). The HeLa cells were treated with 1 μM of BBTA in 2 mL of serum free medium for 2 h and imaged by CLSM without removing the molecule in the cell medium. Confocal fluorescence imaging was performed with Nikon multiphoton microscopy (A1R MP) with a 60× oil-immersion objective lens and living cell work station. The cellular images were taken under a CLSM by using the excitation channel at 488 nm.
1 (HyClone) with 10% Fetal Bovine Serum (FBS) and 1% penicillin–streptomycin) at 37 °C under a humidified atmosphere containing 5% CO2 for 24 h. The cells were seeded on a Ø 35 mm glass-bottomed dish (NEST) for live-cell imaging by confocal laser scanning microscopy (CLSM). The HeLa cells were treated with 1 μM of BBTA in 2 mL of serum free medium for 2 h and imaged by CLSM without removing the molecule in the cell medium. Confocal fluorescence imaging was performed with Nikon multiphoton microscopy (A1R MP) with a 60× oil-immersion objective lens and living cell work station. The cellular images were taken under a CLSM by using the excitation channel at 488 nm.
Toxicity test
Toxicity test of HeLa cells incubated with BBTA is carried out as follows: (a) HeLa cells were incubated with 1 μM of BBTA for 2 h, after washed up 3 times with phosphate buffered saline (PBS), 1 mL of fresh PBS was added. (b) To the incubated HeLa cells in PBS was added propidium iodide (PI) probe, after incubation for 10 min, the HeLa cells with BBTA and PI probe were washed up with PBS for three times, 500 μL of fresh PBS was then added. (c) The sample was observed by Nikon A1R confocal fluorescence microscope with excitation wavelength of 488 nm and 561 nm, respectively, and the range of collected fluorescence is 500–560 nm and 570–620 nm, respectively. (d) The number of dead cells (red) and the whole number of cells were counted from the obtained images. Around 200 cells were counted, and the ratio of living cells (viability, %) was calculated. The viability of the cells without incubation of BBTA was also checked by PI under the same experimental condition. The viability (%) of stained cells is calculated by relation to that of unstained cells in which the viability of unstained cells is set to 100%.Material and methods
1H and 13C NMR spectra are recorded at 400 and 100 MHz, respectively, with TMS as an internal reference. HRMS spectra are recorded with MS spectrometer (Waters, GCT Premier). UV absorption spectra and fluorescence spectra are measured with an absorption spectrophotometer (Hitachi U-3010) and a fluorescence spectrophotometer (F-2500), respectively. All experiments are carried out with commercially available reagents and solvents, and used without further purification, unless otherwise noted.Results and discussion
BBTA was obtained by a two-step reaction starting from BBT, which was nitrified first and reduced followed (Scheme 2). Treatment BBT with nitric acid (65%, wt%) in conc. H2SO4 (98%, wt%) at 0 °C provided crude product 2,5-bis(6-nitrobenzoxazol-2-yl)thiophene in yield of 95%. The crude 2,5-bis(6-nitrobenzoxazol-2-yl)thiophene was then reduced by SnCl2·2H2O in ethanol at 80 °C to yield target compound 2,5-bis(6-amine-benzoxazol-2-yl)thiophene BBTA in 80% isolated yield. The details of procedure and the structure characterization of BBTA see Experimental section.Both absorption and fluorescence of BBTA in different organic solvents were measured. As shown in Table 2, the fluorescence of BBTA depended on both polarity and proticity of solvents. In apolar solvent (toluene) or small polar solvent (DCM), BBTA showed a green emission with the maximum emission around at 500 nm, but in polar solvent (DMSO) or protic solvent (EtOH), an orange emission (558 nm for DMSO, 550 nm for EtOH) was obtained. The large bathochromic shifts of BBTA with polarity or proticity of solvents suggested that BBTA in the excited stated exhibited large polarity, which resulted in more affected by polar solvents or protic solvents. Besides, significant fluorescence quenching was also obtained in large polar solvents or protic solvents, as shown in Table 2, the fluorescence quantum yield (Φf) was decreased from 0.99 in toluene to 0.33 in DMSO or 0.32 in EtOH, which probably resulted from the narrowing of the singlet–triplet energy gap in large polar solvents, and favoured internal conversion58 or intersystem crossing to the triplet state.59
| Solvent | λmax (nm) | εmax (M−1 cm−1) | λem (nm) | Φfd | Δλ (nm) | Δν (cm−1) |
|---|---|---|---|---|---|---|
a C7H8: toluene; MeCN: acetonitrile; EtOH: ethanol; DMSO: dimethyl sulfoxide.b PBS: PBS–mPEG550 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v).c PBS: PBS–DMSO (99 5, v/v).c PBS: PBS–DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).d Φf: fluorescence quantum yield using fluorescein (Φf = 0.95, 0.1 M NaOH) as reference. 1, v/v).d Φf: fluorescence quantum yield using fluorescein (Φf = 0.95, 0.1 M NaOH) as reference. |
||||||
| C7H8 | 405 | 6.6 × 103 | 495 | 0.99 | 90 | 4489 |
| CH2Cl2 | 404 | 1.0 × 104 | 505 | 0.94 | 101 | 4950 |
| MeCN | 406 | 1.4 × 104 | 534 | 0.73 | 128 | 5904 |
| EtOH | 420 | 1.3 × 104 | 550 | 0.30 | 130 | 5627 |
| DMSO | 436 | 1.4 × 104 | 558 | 0.33 | 122 | 5015 |
| PBSb | 382 | 1.2 × 104 | 568 | 0.11 | 186 | 8572 |
| PBSc | 405 | 1.2 × 104 | 568 | 0.13 | 163 | 7085 |
Absorbance and fluorescence of BBTA (20 μM) in PBS buffer solution (NaCl 137 mmol L−1, KCl 2.7 mmol L−1, Na2HPO4 10 mmol L−1, KH2PO4 2 mmol L−1, pH = 7.2) were also measured. To enhance aqueous solubility, 5% (v/v) of polyethylene glycol monomethyl ether (mPEG550, MW = 550) as additive was added. The maximum absorption of BBTA was shifted to λmax = 382 nm with a large extinction coefficient (ε = 1.2 × 104 cm−1 M−1). As compared to the λmax of BBTA in organic solvents (Table 1), a large blue-shift (Δλ ≥ 22 nm) was obtained, which probably resulted from the aggregation of BBTA in PBS because of mPEG550. To confirm the suggestion, the control experiment was performed using PBS replaced PBS–mPEG550. A stock solution of BBTA in DMSO (1 mg mL−1) was prepared and diluted with PBS to 10 μM. It was found that the absorption of BBTA appeared at λmax = 405 nm in PBS solution, which is similar to that in organic solvents. Besides, both absorption and excitation spectral in PBS–mPEG550 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) almost overlapped (Fig. 1), which also indicated that the aggregation was formed in ground state. Upon excitation with 405 nm light, an orange fluorescence (λem = 568 nm) was observed, and a moderate fluorescence quantum yield (Φf = 0.11) was obtained by using fluorescein (Φf = 0.95, 0.1 M NaOH) as reference. As shown in Fig. 1, the overlap between the absorption and fluorescence spectral was very small, and a very large Stokes shift (Δλ = 186 nm, or Δν = 8572 cm−1) was obtained as compared to that of typical commercial fluorescent dyes (Table 1). The large Stokes shift is beneficial to practical application since it can reduce self-quenching that is resulting from molecular self-absorption.
5, v/v) almost overlapped (Fig. 1), which also indicated that the aggregation was formed in ground state. Upon excitation with 405 nm light, an orange fluorescence (λem = 568 nm) was observed, and a moderate fluorescence quantum yield (Φf = 0.11) was obtained by using fluorescein (Φf = 0.95, 0.1 M NaOH) as reference. As shown in Fig. 1, the overlap between the absorption and fluorescence spectral was very small, and a very large Stokes shift (Δλ = 186 nm, or Δν = 8572 cm−1) was obtained as compared to that of typical commercial fluorescent dyes (Table 1). The large Stokes shift is beneficial to practical application since it can reduce self-quenching that is resulting from molecular self-absorption.
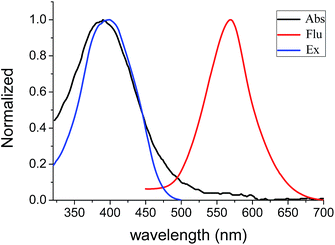 | ||
Fig. 1 Absorption, excitation (20 μM) and fluorescence (10 μM) spectral of BBTA in PBS–mPEG550 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v), λex = 405 nm. 5, v/v), λex = 405 nm. | ||
BBTA applied to fluorescence imaging in living cells was explored. HeLa living cells were incubated with BBTA (1.0 μM) in PBS solution (with 5% of mPEG550) for 2 h, and the images of the live cells were taken by using a confocal laser scanning microscope (CLSM). Upon excitation with 488 nm and recorded at channel (500–550 nm), HeLa cells incubated with BBTA showed fluorescence signal, as shown in Fig. 2, the fluorescence images indicated that BBTA was clearly expressed in HeLa cells. It is worth noting that an enhanced fluorescence was observed when BBTA combined with HeLa living cells, as a consequence, HeLa cells incubated with BBTA could be directly used for microscopic images without washing up by phosphate-buffered saline (PBS). As presented in Fig. 2, no significant background interference was detected when the incubated HeLa cells was used for microscopic images without washing up.
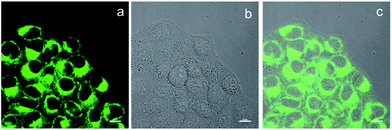 | ||
| Fig. 2 CLSM images of HeLa cells. (a) Fluorescence imaging incubated with BBTA; (b) bright field imaging; (c) merged imaging. Scale bars: 10 μm. | ||
To determine the cellular localization of BBTA, the co-localization experiment with Mito Tracker Deep Red was performed. HeLa cells were incubated with 1.0 μM of BBTA for 2 h, followed by incubation with 25 nM of Mito Tracker Deep Red for 20 min. Both 488 nm and 640 nm excitation wavelength were employed for BBTA and Mito Tracker Deep Red, respectively, and the fluorescence was recorded at channel (500–550 nm) and (670–720 nm), respectively. As presented in Fig. 3, the image with the probe is in good agreement with that of the commercial Mito Tracker Deep Red, and the overlaid confocal fluorescence images of both BBTA and Mito Tracker Deep Red demonstrated that BBTA was expressed in mitochondria.
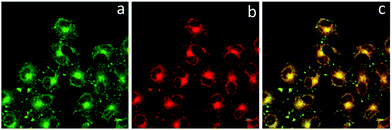 | ||
| Fig. 3 CLSM images of HeLa cells. (a) Fluorescence imaging incubated with BBTA; (b) fluorescence imaging incubated with Mito Tracker Deep Red; (c) merged imaging. Scale bars: 10 μm. | ||
Discrimination against background fluorescence of HeLa cells was also conducted. Both fluorescence imaging from incubated HeLa cells with BBTA (1.0 μM) and from background fluorescence imaging were obtained by using a confocal laser scanning microscope. As is demonstrated in Fig. 4, with excitation at 488 nm and recorded at channel (500–550 nm), both HeLa cells with and without incubation with BBTA showed fluorescence signal, the auto-fluorescence of HeLa cells showed, however, much weaker than that of incubated HeLa cells, and a high contrast in fluorescence imaging was obtained. As shown in Fig. 4, the auto-fluorescence signal was hardly identified after the HeLa cells were incubated with BBTA.
 | ||
| Fig. 4 CLSM images of HeLa cells. (a) Fluorescence imaging incubated with BBTA; (b) auto-fluorescence imaging; (c) merged imaging. Scale bars are 10 μm. | ||
Both pH-sensitivity and photo-stability are important features of a probe used for biological applications. pH-Sensitivity of BBTA was performed in H2O–mPEG550 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution by adjusting with HCl or NaOH aqueous solution, respectively. As displayed in Fig. 5, no distinct fluorescence quenching was observed at emission peak (detection at λem = 568 nm) with different pH range from 2 to 10. Besides, it was also found that no obvious change in both absorption spectral (wavelength and optical density) and emission wavelength was detected when the pH of the solution changed from 2 to 10, which demonstrated that BBTA is stable in aqueous solution over a wide pH range, and beneficial for potential bioimaging application in physiological environments.
5, v/v) solution by adjusting with HCl or NaOH aqueous solution, respectively. As displayed in Fig. 5, no distinct fluorescence quenching was observed at emission peak (detection at λem = 568 nm) with different pH range from 2 to 10. Besides, it was also found that no obvious change in both absorption spectral (wavelength and optical density) and emission wavelength was detected when the pH of the solution changed from 2 to 10, which demonstrated that BBTA is stable in aqueous solution over a wide pH range, and beneficial for potential bioimaging application in physiological environments.
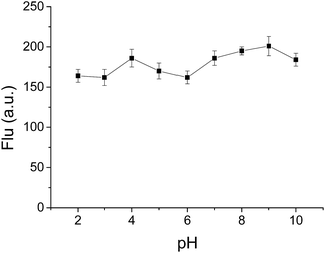 | ||
Fig. 5 Fluorescence intensity of BBTA in H2O–mPEG550 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v, 10 μM) with different pH (error bar represents standard deviation). λex = 405 nm. 5, v/v, 10 μM) with different pH (error bar represents standard deviation). λex = 405 nm. | ||
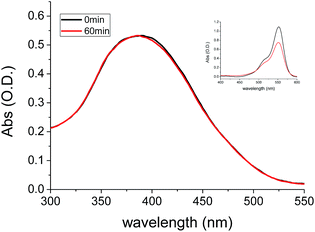
The photo-stability of BBTA was investigated in both PBS–mPEG550 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) aqueous solution and living cells, respectively. Fig. 6 represented the absorption changes of BBTA in PBS–mPEG330 (95
5, v/v) aqueous solution and living cells, respectively. Fig. 6 represented the absorption changes of BBTA in PBS–mPEG330 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) aqueous solution as a function of irradiating time with 365 nm light (power: 36 W, energy: 3.7 mW cm−2). It was found that no significant change in optical density at λmax = 382 nm was detected when the BBTA solution was irradiated for 60 min, as shown in Fig. 6, less than 0.1% of degradation was detected (calculation by the change in optical density). To compare the photostability, a commercial fluorescent organic dye rhodamine B was employed for control experiment. As shown in Fig. 6 (inset) more than 30% degradation was obtained upon irradiation of rhodamine B in PBS–mPEG550 (95
5, v/v) aqueous solution as a function of irradiating time with 365 nm light (power: 36 W, energy: 3.7 mW cm−2). It was found that no significant change in optical density at λmax = 382 nm was detected when the BBTA solution was irradiated for 60 min, as shown in Fig. 6, less than 0.1% of degradation was detected (calculation by the change in optical density). To compare the photostability, a commercial fluorescent organic dye rhodamine B was employed for control experiment. As shown in Fig. 6 (inset) more than 30% degradation was obtained upon irradiation of rhodamine B in PBS–mPEG550 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution for 60 min. This suggested that the BBTA showed excellent photo-degradation resistance. Meanwhile, no significant change in fluorescence intensity was observed when the solution was irradiated for 60 min under 365 nm light.
5, v/v) solution for 60 min. This suggested that the BBTA showed excellent photo-degradation resistance. Meanwhile, no significant change in fluorescence intensity was observed when the solution was irradiated for 60 min under 365 nm light.
The photo-stability of BBTA in living cells was explored by continuous laser exposure using confocal laser scanning microscopy. After scanning for 10 min, no significant change in the CLSM images was obtained (Fig. 7), which indicated that BBTA exhibits high photo-stability for bioimaging.
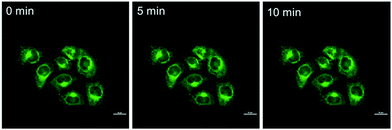 | ||
| Fig. 7 CLSM images of HeLa cells incubated with BBTA with increasing scanning times (0–10 min). Scale bars: 10 μm. | ||
The high photo-stability of BBTA probably results from oxygen depletion. It is known that the rate of triplet state quenching by molecular oxygen is faster than the formation of radical states in the absence of high concentrations of oxidants or reductants. The fluorescence of BBTA in PBS was much smaller than that in organic solvents such as toluene or CH2Cl2. The major competitive fluorescence quenching pathway of 2,5-bis(benzoxazol-2-yl)thiophene unit was demonstrated60 by intersystem crossing to the triplet state. Oxygen consumption by triplet state may benefit to photostability of BBTA.
Toxicity is an important factor to evaluate the application possibility of fluorescence dyes. To test the cytotoxicity of BBTA, propidium iodide (PI, Invitrogen, P3566), which is widely used in the toxicity study for identifying dead cells in a population, was employed as the probe for the detection of dead cells of HeLa. The HeLa cells incubated with BBTA and PI probe were excited by 488 nm and 561 nm, respectively, and observed by Nikon A1R confocal fluorescence microscope with the fluorescence recorded at channel (500–550 nm) and (570–620 nm), respectively. The number of dead cells and the whole number of cells were counted from the obtained images, and the viability (%) (the ratio of living cells) was calculated by the comparison of the number of living cells with that of the dead cells. As shown in Fig. 8, more than 98% of viability was obtained when the HeLa cells were incubated with BBTA (1.0 μM) within 2 h. The preliminary result indicated that BBTA showed low cytotoxicity at the concentration used for cell imaging.
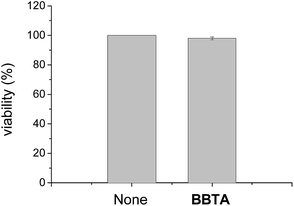 | ||
| Fig. 8 The viability of HeLa cells with (right column) and without (left column) incubation with BBTA (1.0 μM) for 2 h (error bar represents standard deviation). | ||
Conclusions
In summary, a new fluorescent organic dye based on 2,5-bis(benzoxazol-2-yl)thiophene derivative has been developed and its application to living cells imaging has been demonstrated. The fluorescence dye has some distinct advantages including facile preparation, high yield, large Stokes shift and excellent photo-stability, which benefits the biological fluorescence imaging.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21572241 and No. 61475164).Notes and references
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2014, 9, 855 CrossRef CAS PubMed.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142 CrossRef CAS PubMed.
- K. Li, W. Qin, D. Ding, N. Tomczak, J. Geng, R. Liu, J. Liu, X. Zhang, H. Liu, B. Liu and B. Z. Tang, Sci. Rep., 2013, 3, 1150 Search PubMed.
- X. Wu, X. Sun, Z. Guo, J. Tang, Y. Shen, T. D. James, H. Tian and W. Zhu, J. Am. Chem. Soc., 2014, 136, 3579 CrossRef CAS PubMed.
- J. F. Araneda, W. E. Piers, B. Heyne, M. Parvez and R. McDonald, Angew. Chem., Int. Ed., 2011, 50, 12214 CrossRef CAS PubMed.
- D. M. Shcherbakova, M. A. Hink, L. Joosen, T. W. J. Gadella and V. V. Verkhusha, J. Am. Chem. Soc., 2012, 134, 7913 CrossRef CAS PubMed.
- Q. Ye, S. Chen, D. Zhu, X. Lu and Q. Lu, J. Mater. Chem. B, 2015, 3, 3091 RSC.
- Z. Wang, S. Chen, J. W. Y. Lam, W. Qin, R. T. K. Kwok, N. Xie, Q. Hu and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 8238 CrossRef CAS PubMed.
- J. K. Jaiswal, H. Mattoussi, J. M. Mauro and S. M. Simon, Nat. Biotechnol., 2002, 21, 47 CrossRef PubMed.
- Y. Duan, M. Liu, W. Sun, M. Wang, S. Liu and Q. X. Li, Mini-Rev. Org. Chem., 2009, 6, 35 CrossRef CAS.
- M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC.
- A. P. Gorka, R. R. Nani and M. J. Schnermann, Org. Biomol. Chem., 2015, 13, 7584 CAS.
- J. L. Bricks, A. D. Kachkovshii, Y. L. Slominskii, A. O. Gerasov and S. V. Popov, Dyes Pigm., 2015, 121, 238 CrossRef CAS.
- M. Henary and A. Levitz, Dyes Pigm., 2013, 99, 1107 CrossRef CAS.
- M. Panigrahi, S. Dash, S. Patel and B. K. Mishra, Tetrahedron, 2012, 68, 781 CrossRef CAS.
- A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973 CrossRef CAS PubMed.
- M. Levitus and S. Ranjit, Q. Rev. Biophys., 2011, 44, 123 CrossRef CAS PubMed.
- J. Jose and K. Burgess, Tetrahedron, 2006, 62, 11021 CrossRef CAS.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed.
- Y. Ni and J. Wu, Org. Biomol. Chem., 2014, 12, 3774 CAS.
- Y. Hayashi, N. Obata, M. Tamaru, S. Yamaguchi, Y. Matsuo, A. Saeki, S. Seki, Y. Kureishi, S. Saito, S. Yamaguchi and H. Shinokubo, Org. Lett., 2012, 14, 866 CrossRef CAS PubMed.
- B. K. Nunnally, H. He, L.-C. Li, S. A. Tucker and L. B. McGown, Anal. Chem., 1997, 69, 2392 CrossRef CAS PubMed.
- K. Jia, Y. Wan, A. Xia, S. Li, F. Gong and G. Yang, J. Phys. Chem. A, 2007, 111, 1593 CrossRef CAS PubMed.
- M. M. Bishop, J. D. Roscioli, S. Ghosh, J. J. Mueller, N. C. Shepherd and W. F. Beck, J. Phys. Chem. B, 2015, 119, 6905 CrossRef CAS PubMed.
- M. Vendrell, D. Zhai, J. C. Er and Y. Chang, Chem. Rev., 2012, 112, 4391 CrossRef CAS PubMed.
- X. Wang and O. S. Wolfbeis, Chem. Soc. Rev., 2014, 43, 3665 Search PubMed.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC.
- Q. Huaulmé, M. Mirloup, P. Retailleau and R. Ziessel, Org. Lett., 2015, 17, 2246 CrossRef PubMed.
- X. Wu and W. Zhu, Chem. Soc. Rev., 2015, 44, 4179 RSC.
- Q. Zhang, M. F. Juette, S. Jockusch, M. R. Wasserman, Z. Zhou, R. B. Altmana and S. C. Blanchard, Chem. Soc. Rev., 2014, 43, 1044 RSC.
- J. Jose and K. Burgess, J. Org. Chem., 2006, 71, 7835 CrossRef CAS PubMed.
- D. T. Reilly, S. H. Kim, J. A. Katzenellenbogen and C. M. Schroeder, Anal. Chem., 2015, 87, 11048 CrossRef CAS PubMed.
- Y. Yang, X. Wang, Q. Cui, Q. Cao and L. Li, ACS Appl. Mater. Interfaces, 2016, 8, 7440 CAS.
- A. Pedone, G. Prampolini, S. Monti and V. Barone, Chem. Mater., 2011, 23, 5016 CrossRef CAS.
- Y. Zhong, F. Peng, F. Bao, S. Wang, X. Ji, L. Yang, Y. Su, S.-T. Lee and Y. He, J. Am. Chem. Soc., 2013, 135, 8350 CrossRef CAS PubMed.
- J. E. Donehue, E. Wertz, C. N. Talicska and J. S. Biteen, J. Phys. Chem. C, 2014, 118, 15027 CAS.
- C.-A. J. Lin, T.-Y. Yang, C.-H. Lee, S. H. Huang, R. A. Sperling, M. Zanella, J. K. Li, J.-L. Shen, H.-H. Wang, H.-I. Yeh, W. J. Parak and W. H. Chang, ACS Nano, 2009, 3, 395 CrossRef CAS PubMed.
- C. Wu, Y. Zheng, C. Szymanski and J. McNeill, J. Phys. Chem. C, 2008, 112, 1772 CAS.
- Z. Tian, J. Yu, X. Wang, L. C. Groff, J. L. Grimland and J. D. McNeill, J. Phys. Chem. B, 2013, 117, 4517 CrossRef CAS PubMed.
- C. Jiao, K.-W. Huang and J. Wu, Org. Lett., 2011, 13, 632 CrossRef CAS PubMed.
- N. I. Shank, H. H. Pham, A. S. Waggoner and B. A. Armitage, J. Am. Chem. Soc., 2013, 135, 242 CrossRef CAS PubMed.
- T. Inari, M. Yamano, A. Hirano, K. Sugawa and J. Otsuki, J. Phys. Chem. A, 2014, 118, 5178 CrossRef CAS PubMed.
- Z. Yuan, A. H. Younes, J. R. Allen, M. W. Davidson and L. Zhu, J. Org. Chem., 2015, 80, 5600 CrossRef CAS PubMed.
- W. Liu, B. Zhou, G. Niu, J. Ge, J. Wu, H. Zhang, H. Xu and P. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 7421 CAS.
- K. Seth, S. K. Garg, R. Kumar, P. Purohit, V. S. Meena, R. Goyal, U. C. Banerjee and A. K. Chakraborti, ACS Med. Chem. Lett., 2014, 5, 512 CrossRef CAS PubMed.
- Y. Sato, M. Yamada, S. Yoshida, T. Soneda, M. Ishikawa, T. Nizato, K. Suzuki and F. Konno, J. Med. Chem., 1998, 41, 3015 CrossRef CAS PubMed.
- S. K. Gorla, M. Kavitha, M. Zhang, J. E. W. Chin, X. Liu, B. Striepen, M. Makowska-Grzyska, Y. Kim, A. Joachimiak, L. Hedstrom and G. D. Cuny, J. Med. Chem., 2013, 56, 4028 CrossRef CAS PubMed.
- M. Calle, C. M. Doherty, A. J. Hill and Y. M. Lee, Macromolecules, 2013, 46, 8179 CrossRef CAS.
- T. Agag, J. Liu, R. Graf, H. W. Spiess and H. Ishida, Macromolecules, 2012, 45, 8991 CrossRef CAS.
- H. A. Patel, D. Ko and C. T. Yavuz, Chem. Mater., 2014, 26, 6729 CrossRef CAS.
- I. M. Ward, in Structure and properties of oriented polymers, Applied Science Publishers, London, 1975, 1st edn, pp. 1–500 Search PubMed.
- M. O. Liu, H. F. Lin, M. C. Yang, M. J. Lai, C. C. Chang, H. C. Liu, P. L. Shiao, I. M. Chen and J. Y. Chen, Mater. Lett., 2006, 60, 2132 CrossRef CAS.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed.
- U. Mitschke and P. BaÈuerle, J. Mater. Chem., 2000, 10, 1471 RSC.
- Y. Hao, M. Zheng and Y. Chen, J. Mater. Chem. B, 2014, 2, 7369 RSC.
- Application number: JP 1991-219950. Full text is accessible through: https://www.j-platpat.inpit.go.jp/web/all/top/BTmTopEnglishPage.
- F. Shibahara, E. Yamaguchi and T. Murai, Chem. Commun., 2010, 46, 2471 RSC.
- Y. Liu, M. Nishiura, Y. Wang and Z. Hou, J. Am. Chem. Soc., 2006, 128, 5592 CrossRef CAS PubMed.
- M. V. Skorobogatyi, A. A. Pchelintseva, A. L. Petrunina, I. A. Stepanova, V. L. Andronova, G. A. Galegov, A. D. Malakhov and V. A. Korshun, Tetrahedron, 2006, 62, 1279 CrossRef CAS.
- M. A. Fourati, T. Maris, W. G. Skene, C. G. Bazuin and R. E. Prud'homme, J. Phys. Chem. B, 2011, 115, 12362 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |