G-quadruplex/protoporphyrin IX-functionalized silver nanoconjugates for targeted cancer cell photodynamic therapy†
Jun Ai‡
ac,
Jing Li‡c,
Lu Gab,
Guohong Yuna,
Li Xuc and
Erkang Wang*c
aCollege of Chemistry and Enviromental Science, Inner Mongolia Normal University, 81 Zhaowudalu, Hohhot 010022, China
bCollege of Pharmacy, Inner Mongolia Medical University, Jinchuankaifaqu, Hohhot 010110, China
cState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin, China. E-mail: ekwang@ciac.ac.cn
First published on 23rd September 2016
Abstract
A new type of G-quadruplex/protoporphyrin IX-functionalized silver nanoconjugate was prepared and used for the targeted photodynamic therapy of cancer cells via the specific interaction between AS1411 and the nucleolin on the cell surface.
Photodynamic therapy (PDT) is an attractive and useful medical tool for the treatment of malignant tissues.1 In PDT, an efficient photosensitizer with high quantum yields of reactive oxygen species (ROS) under the irradiation of red or near-infrared light is desirable. Protoporphyrin IX (PPIX) is one of the most well-known example of photodynamic agents in cancer therapy due to the unique photophysical features.2 However, the poor water solubility of PPIX impairs its systemic biodistribution and reduces the quantum yields of ROS.3 On the other side, the limited tumor or cancer cell localization of pure PPIX lead to the photodamage of peripheral tissue. Recently, it was reported that the G-quadruplex DNAs can bind free PPIX with high affinity, which can remarkably enhance the fluorescence of PPIX.4 Moreover, they themselves can also be acted as active anti proliferative agents. For example, AS1411, a 26-base guanine-rich oligonucleotide, currently has been used as an anticancer agent under phase II clinical trials, which represents the first nucleic acid-based aptamer tested for the treatment of cancer in humans.5 Of particular, G-quadruplex DNAs can bind the target nucleolin receptors over-expressed on the cancer cells specifically.6 Therefore, G-quadruplex DNA should hold a great promise as the attractive carrier of the PPIX and the recognized elements in the targeted cancer cell PDT.
Silver nanoparticles (AgNPs) with unique optical, electronic, and antibacterial properties have received considerable interest in bioanalysis, diagnosis, and biomedical treatment.7 Recently, AgNPs has been introduced to the therapeutic applications as promising anticancer agents.8 Enhanced performance has been obtained when AgNPs was used for cancer treatments. For instance, Gurunathan et al. used the biologically synthesized AgNPs to induce the cell death of breast cancer via the ROS generation, activation of caspase 3 and DNA fragmentation.9
Inspired by the favorable feature of G-quadruplex DNAs and AgNPs, a synergistic nanoplatform based on the G-quadruplex DNA/PPIX-functionalized AgNPs (termed as PAAs) was fabricated for the targeted cancer cell PDT. Here, AS1411 was chosen for constructing the G-quadruplex DNA in the presence of K+. The nanocarrier of AgNPs with easy ability to conjugate with anti-cancer drugs was first prepared using the glutathione as protecting agent. The AgNPs was further functionalized with SH-AS1411 for cancer-specific PDT via the Ag–S bond. In the presence of the K+, G-quadruplex DNA structure was formed and provided the anchoring sites for the photosensitizer. Due to the specific interaction (AS1411-nucleolin) and synergistic effect of the PPIX and AgNPs, targeted PDT using multifunctional nanoconjugates was achieved with high performance (Scheme 1).
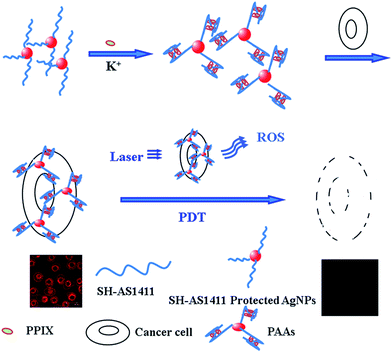 | ||
| Scheme 1 Principle of the targeted cell imaging and PDT to cancer cells over-expressed with nucleolin on the cell surface using PAAs. | ||
Transmission electron microscope (TEM) was employed to characterize the size and morphology of the prepared AgNPs. It is clearly seen that monodispersed AgNPs with the diameter of 5 nm was obtained (Fig. 1A). Due to the Ag–S bond, SH-AS1411 could easily assemble on the surface of AgNPs. In the presence of 100 mM K+, circular dichroism (CD) experiments were carried out to identify the formed quadruplex structures. As shown in Fig. 1B, the positive peak at 264 nm and negative peak at 240 nm were observed using both SH-AS1411 and PAAs, which indicated the formation of parallel quadruplex structures.10 After incubation with PPIX (Fig. 1B), a remarkably fluorescence enhancement was observed due to the formation of the G-quadruplex DNAs.4 It should be noted that a slight decrease of fluorescence was observed compared with SH-AS1411/PPIX, which may be attributed to the fluorescence quenching induced by the AgNPs.11 Here, the AgNPs with the maximum plasmon absorption band at ca. 420 nm (see ESI Fig. S1†) function as an absorber of the excitation of light and induced the less light available for the excitation of PPIX.
Owing to its favorable spectroscopic properties of the PAAs, fluorescence imaging in living tumor cells was investigated. HeLa cells (nucleolin overexpressed cancer cell) were incubated with a 10 μM solution of PAAs in PBS buffer for 2 h at 37 °C and 5% CO2, followed by recording the image with laser scanning confocal microscope. The bright red of fluorescence from the intracellular area appeared due to the cellular uptake of PAAs (Fig. 2A). As a control, the HEK 293 cells (normal cells) was employed and stained with PAAs for 2 h. No distinct fluorescence was present, indicating the targeted ability of the PAAs caused by the specific binding between AS1411 and the nucleolin on the cell surface.12 AFM characterizations of the morphology of cancer cells incubated with PAAs further confirmed the cellular uptake of PAAs mediated by nucleolin (shown in Fig. S3†). Therefore, PAAs nanoconjugates can be used for the efficient PDT. To evaluate the PDT efficacy of the PAAs, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) cell viability assays were carried out by varying concentrations. Non-transfected cells were severed as the control and the cell viability of which was set as 100%. As shown in Fig. 3B, the AgNPs, SH-AS1411 and PAAs showed only minor cytotoxicity in the dark. In contrast, under the light illumination for 30 min, it is clear from Fig. 3C that the PAAs exhibited obvious cytotoxicity due to the production of the singlet oxygen [ESI Fig. S2†]. Compared with AS1411/PPIX complex, enhanced photodynamic efficacy was achieved when G-quadruplex/PPIX was grafted on the nanoparticles for the targeted PDT. And the lower concentration of the PAAs (e.g. 50 nM) resulted in the remarkably cytotoxicity suggesting the synergistic effect between G-quadruplex DNAs and AgNPs. P values of p < 0.05 were considered statistically significant. All results described above highlighted the effectiveness of PAAs for specifically targeting cancerous cells for efficient cancer therapy.
 | ||
| Fig. 2 Fluorescence imaging of HeLa cell (A) and HEK293 cell (B) incubated with PAAs at 37 °C and 5% CO2. | ||
In summary, G-quadruplex/PPIX-functionalized fluorescence silver nanoconjugates was found to have a promising toxicity profile for photodynamic cancer therapy. AgNPs with the ability of easy assembly with anti-cancer drugs was employed as nanocarrier. The G-quadruplex was formed using AS1411 in the presence of KCl, which can be used for capturing and delivering the photosensitizer of PPIX with enhanced fluorescence efficiently. Meanwhile, the AS1411 anchored on the surface of AgNPs can specifically recognize and bind to the nucleolin in living HeLa cells for achieving the targeted photodynamic cancer therapy. Compared with the AgNPs and AS1411/PPIX complex, targeted nanoconjugates exhibited enhanced photodynamic cancer therapy efficiency due to the synergistic effect. The ability to target this cell-surface nucleolin and efficiently induce the cell death using PAAs makes them promising materials for the targeted photodynamic cancer therapy.
Experimental
Materials
Tris(β-chloroethyl) phosphate (TCEP) was purchased from Fluka (Buchs, Switzerland). Glutathione and all chemicals of analytical grade were used as received without further purification. The oligodeoxynucleotides used in the present study were SH-AS1411, an antiproliferative G-rich oligodeoxynucleotides, whose sequence is 5′-d(GGTGGTGGTGGTTGTGGTGGTGGTGGTTTSH)-3′, and were synthesized by Shanghai Sangon Biotechnology Co. Ltd. (Shanghai, China). 3-[4,5-Dimethylthiazolyl-2]-2,5-diphenyltetrazolium bromide were obtained from Sigma-Aldrich (USA). Phosphate buffered solution (PBS) were prepared by 10 mM phosphate (NaH2PO4 and Na2HPO4), pH = 7.4. The PBS buffer was used to rinsing suspension of HeLa cells. All the solutions were prepared by using distilled water and stored at 4 °C before use. HeLa cells were obtained from the American Type Culture Collection (Manassas, VA) and HEK 293 were obtained from the cancer institute & hospital (Chinese academy of medical school). These cells were maintained in DMEM supplemented with 10% standard fetal bovine serum (Defined FBS) (HyClone Laboratories, UT) at 37 °C and in 5% CO2. Glass chamber slides (14 mm bottom well) were purchased from Hangzhou Sanyou Biotechnology Co. Ltd. (Hangzhou, China).Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21190040 and 11072104), the Natural Science Foundation of Inner Mongolia (Grant no. 2013MS0217), the Program of Higher-level talents of Inner Mongolia University (Grant No. 135118). This research work was supported by the Open Funds of the State Key Laboratory of Electroanalytical Chemistry (SKLEAC201503) and Jilin Province Science and Technology Development Plan Project 20150520082JH.Notes and references
- (a) G. Obaid, M. Broekgaarden, A.-L. Bulin, H.-C. Huang, J. Kuriakose, J. Liu and T. Hasan, Nanoscale, 2016, 8, 12471 RSC; (b) C. Wu, A. Zhu, D. Li, L. Wang, H. Yang, H. Zeng and Y. Liu, Expert Opin. Drug Delivery, 2016, 13, 155 CrossRef CAS PubMed; (c) A. El-Hussein, I. Mfouo-Tynga, M. Abdel-Harith and H. Abrahamse, J. Photochem. Photobiol., B, 2015, 153, 67 CrossRef CAS PubMed; (d) M. Nafiujjaman, M. Nurunnabi, S.-H. Kang, G. R. Reeck, H. A. Khan and Y.-K. Lee, J. Mater. Chem. B, 2015, 3, 5815 RSC; (e) K. Yang, L. Feng, X. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530 RSC; (f) L. Feng, L. Wu and X. Qu, Adv. Mater., 2013, 25, 168 CrossRef CAS PubMed.
- (a) P. Palasuberniam, X. Yang, D. Kraus, P. Jones, K. A. Myers and B. Chen, Sci. Rep., 2015, 5, 13298 CrossRef CAS PubMed; (b) W. P. Savarimuthu, P. Gananathan, A. P. Rao, E. Manickam and G. Singaravelu, J. Nanosci. Nanotechnol., 2015, 15, 5577–5584 CrossRef CAS PubMed.
- (a) G. Obaid, I. Chambrier, M. J. Cook and D. A. Russell, Angew. Chem., Int. Ed., 2012, 51, 6158–6162 CrossRef CAS PubMed; (b) Z. Zhou, D. Li, L. Zhang, E. Wang and S. Dong, Talanta, 2015, 134, 298–304 CrossRef CAS PubMed.
- (a) J. Zhu, L. Zhang, Z. Zhou, S. Dong and E. Wang, Anal. Chem., 2014, 86, 312–316 CrossRef CAS PubMed; (b) Z. Zhang, E. Sharon, R. Freeman, X. Liu and I. Willner, Anal. Chem., 2012, 84, 4789–4797 CrossRef CAS PubMed; (c) Y. Lv, Q. Xue, X. Gu, S. Zhang and J. Liu, Analyst, 2014, 139, 2583–2588 RSC.
- L. Q. Chen, S. J. Xiao, L. Peng, T. Wu, J. Ling, Y. F. Li and C. Z. Huang, J. Phys. Chem. B, 2010, 114, 3655 CrossRef CAS PubMed.
- (a) V. Brázda, L. Hároníková, J. C. Liao and M. Fojta, Int. J. Mol. Sci., 2014, 15, 17493–17517 CrossRef PubMed; (b) C. Cao, J. Zhang, X. Wen, S. L. Dodson, N. T. Dao, L. M. Wong, S. Wang, S. Li, A. T. Phan and Q. Xiong, ACS Nano, 2013, 7, 7583–7591 CrossRef CAS PubMed.
- L. Wei, J. Lu, H. Xu, A. Patel, Z. S. Chen and G. Chen, Drug Discovery Today, 2015, 20, 595–601 CrossRef CAS PubMed.
- (a) A. M. Goodman, Y. Cao, C. Urban, O. Neumann, C. Ayala-Orozco, M. W. Knight, A. Joshi, P. Nordlander and N. J. Halas, ACS Nano, 2014, 8, 3222–3231 CrossRef CAS PubMed; (b) C. Rajkuberan, K. Sudha, G. Sathishkumar and S. Sivaramakrishnan, Spectrochim. Acta, Part A, 2015, 136, 924–930 CrossRef CAS PubMed; (c) C. A. dos Santos, M. M. Seckler, A. P. Ingle, I. Gupta, S. Galdiero, M. Galdiero, A. Gade and M. Rai, J. Pharm. Sci., 2014, 103, 1931–1944 CrossRef CAS PubMed.
- S. Gurunathan, J. W. Han, V. Eppakayala, M. Jeyaraj and J. H. Kim, BioMed Res. Int., 2013, 2013, 535796 Search PubMed.
- (a) Z. Bagheri, B. Ranjbar, H. Latifi, M. I. Zibaii, T. T. Moghadam and A. Azizi, Int. J. Biol. Macromol., 2015, 72, 806–811 CrossRef CAS PubMed; (b) T. Y. Tseng, Z. F. Wang, C. H. Chien and T. C. Chang, Nucleic Acids Res., 2012, 40, 8711–8720 CrossRef PubMed.
- (a) A. Aguila and R. W. Murray, Langmuir, 2000, 16, 5949–5954 CrossRef CAS; (b) J. Hu, J. Zhang, F. Liu, K. Kittredge, J. K. Whitesell and M. A. Fox, J. Am. Chem. Soc., 2001, 123, 1464–1470 CrossRef CAS; (c) B. I. Ipe, K. G. Thomas, S. Barazzouk, S. Hotchandani and P. V. Kamat, J. Phys. Chem. B, 2002, 106, 18–21 CrossRef CAS; (d) T. Huang and R. W. Murray, Langmuir, 2002, 18, 7077–7081 CrossRef CAS; (e) T. Gu, J. K. Whitesell and M. A. Fox, Chem. Mater., 2003, 15, 1358–1366 CrossRef CAS; (f) M. Montalti, L. Prodi, N. Zaccheroni and G. Battistini, Langmuir, 2004, 20, 7884–7886 CrossRef CAS PubMed; (g) M. H. V. Werts, H. Zaim and M. Blanchard-Desce, Photochem. Photobiol. Sci., 2004, 3, 29–32 RSC; (h) Z. Gueroui and A. Libchaber, Phys. Rev. Lett., 2004, 93, 166108–166111 CrossRef PubMed; (i) S. K. Ghosh, A. Pal, S. Kundu, S. Nath and T. Pal, Chem. Phys. Lett., 2004, 395, 366–372 CrossRef CAS; (j) E. Dulkeith, M. Ringler, T. A. Klar, J. Feldmann, A. M. Javier and W. J. Parak, Nano Lett., 2005, 5, 585–589 CrossRef CAS PubMed.
- M. T. Malik, M. G. O'Toole, L. K. Casson, S. D. Thomas, G. T. Bardi, E. M. Reyes-Reyes, C. K. Ng, K. A. Kang and P. J. Bates, Oncotarget, 2015, 6, 22270–22281 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: A detailed description of Experimental sections are provided. See DOI: 10.1039/c6ra18178c |
| ‡ Jun Ai and Jing Li contributed equally to this work contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |


