Humidity sensing properties of transferable polyaniline thin films formed at the air–water interface
Tong-Fei Wu and
Jong-Dal Hong*
Department of Chemistry, Research Institute of Natural Sciences, Incheon National University, 119 Academy-ro Yeonsu-gu, Incheon, 21022, Republic of Korea. E-mail: hong5506@inu.ac.kr
First published on 5th October 2016
Abstract
This article describes a simple method to prepare transferable polyaniline (PANI) thin films at the air–water interface. The method allows the convenient fabrication of dense and uniform PANI films at the nanoscale, and features high device performance of humidity sensors. The oxidation state of the interfacial PANI films (which closely relies on the conductivity of PANI films) could be optimized by controlling the concentration of aniline in the interfacial. The interfacial PANI thin film prepared from 0.04 M aniline solution (denoted the PANI-0.04 film) was employed for the evaluation of the sensitivity of the thin-film humidity sensor, resulting in 550% change in resistance with relative humidity (RH) ranging from 0% to 100%. The PANI-0.04 film sensor exhibited response (5–7 s) and recovery times (4–7 s) under dynamic tests, which correspond to excellent performance data among the PANI-based humidity sensor systems reported previously. Furthermore, the sensitivity of the PANI-0.04 film could be increased to 1900% and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000%, when the PANI-0.04 film was doped with acetic acid and hydrogen chloride, respectively.
000%, when the PANI-0.04 film was doped with acetic acid and hydrogen chloride, respectively.
Introduction
Humidity plays an important role in technology and human activities including moisture sensitive products, food processing, textile technology, storage areas, computer rooms, hospitals, museums, libraries, high voltage engineering and accelerator systems.1 A number of attempts have been made to design various types of humidity sensors based on semiconductors, conducting polymers and polymer composites, which show various capacitive, resistive, thermal-conductive or optical behaviors in response to relative moisture levels.2,3 Humidity sensors employed today in a wide range of applications for monitoring and controlling of humidity tend to require outstanding moisture-responsive performances of the devices including higher sensitivity, wider humidity detection range, quicker response, and shorter recovery times.Polyaniline (PANI) is one of the most widely studied conducting polymers, because it has many advantages, such as its ease of preparation, low cost monomer, tuneable properties, and environmental sustainability.4 During the last decades, PANI-based materials have shown a great deal of promise in various application areas including electrical and optoelectronic devices, corrosion protection layers, chemical sensors, electromagnetic shielding, antistatic coatings, batteries electrodes, and antibacterial coatings.5–8 The electrical properties of PANI can reversibly be controlled by changing the oxidation state of main chain and by the protonation of amine nitrogen chain,9 because the conductivity of PANI relies on both the transport ability of charge carriers along the polymer backbone and hop of charge carriers between polymer chains, as well as the concentration of charge carriers. Therefore, the alteration in any of these factors will affect the conductivity of polyaniline. For instance, water content on PANI can be attributed to improving the charge transports, resulting in the increased conductivity of PANI, which is benefit to the realization of PANI-based humidity sensors.10–14 Four orders of magnitude change in impedance was observed from humidity sensors based on electrospun PANI–poly(styrene sulfonic acid) nanofibers for RH change from 20% to 90%.11 In another study, resistive humidity sensors based on PANI nanofibers exhibited high sensitivity of up to 291% variation in resistance for RH change from 0% to 100%.12 An additional advantage of PANI in the use of humidity sensing is easy solution process for the preparation of various material features including nanofibers, flakes, and thin films, which were produced during the oxidative polymerization of aniline.15–20 Thin films of PANI are considered to be ideal building blocks in miniature sensing devices owing to the high ratio of surface area to volume. A number of methods have been developed to fabricate thin films of PANI through electropolymerization,21 spinning coating,22 solution casting,23 and Langmuir–Blodgett technique.24–27 From a technical point of view, transferable thin films of PANI could strongly simplify the fabrication process for humidity sensing devices. One of the preparation methods for transferable thin films is the interfacial assembly method, which has been applied for mesostructured thin films of inorganic oxides and the polydopamine thin films.28,29 The thin film formation at an air–water interface was attributed in the hydrophobicity of materials and the mass transport of aggregates through the convective flow (caused by water evaporation),30 which drives the aggregates trapped and accumulated at the interface. PANI was also found being able to form thin films with two-dimensional mesoscopic and macroscopic patterns at air–water interface through a Belousov–Zhabotinsky (BZ)-reaction-coupled polymerization of aniline.31 And this process could be further simplified by using the standard aniline oxidative polymerization in the presence of hydrochloric acid and ammonium persulfate in capped crystallization dishes under static conditions.32
This article described the development of a high performance humidity sensor fabricated with the PANI thin films formed at air–water interface through a BZ-reaction-coupled polymerization of aniline.31 Thereby, the interfacial PANI films were doped with different acid doping agents, i.e. acetic acid and hydrogen chloride, which are supposed to affect the humidity sensing performance. It's well known that PANI exhibits different conducting behaviors, as doped with different acids.33 The interfacial PANI thin films were prepared through the oxidative polymerization of aniline at an air/water interface, as reported previously.32 Then, the as-prepared thin film was subject to the doping agents; acetic acid or hydrogen chloride vapors. The morphology of the interfacial PANI films was investigated using field-emission scanning electron microscopy (FE-SEM). The chemical structures of PANI thin films were also investigated using UV-visible spectroscopy (UV-vis) and Fourier transform infrared spectroscopy (FTIR). The humidity sensing properties of the interfacial PANI films on interdigital gold electrodes was evaluated based on the variation of the conductance in response to RH levels under static and dynamic stimuli.
Experimental
Materials
Aniline (99.5%) and ammonium persulfate (APS, 98%) were purchased from Sigma-Aldrich, and used as received. Acetic acid (HAc) (99.5%, DC Chemical), hydrogen chloride (HCl) (35–37%, SAMCHUN), magnesium chloride hexahydrate (MgCl2·6H2O) (98%, OCI), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) (98%, DAEJUNG), sodium chloride (NaCl) (99.5%, DAEJUNG), potassium chromate (K2CrO4) (99%, DAEJUNG), and calcium chloride anhydrous (CaCl2) (96%, SAMCHUN) were used as received.Preparation of transferable polyaniline (PANI) thin films
The PANI samples were obtained by the oxidation of aniline using APS at room temperature (RT).34 Two milliliter of aqueous aniline solutions (whose concentrations were ranged from 0.01 M, 0.02 M, 0.04 M, to 0.08 M, respectively) was mixed with the 1.25-fold amount of APS. The mixture was poured into plastic Petri dishes (35 mm × 10 mm), which were put with the lids afterward. The PANI thin films were formed at air–water interface, as the aniline/APS mixture solution was kept for 24 h under static condition at RT. The interfacial PANI films were denoted to PANI-0.01, PANI-0.02, PANI-0.04, and PANI-0.08, respectively, corresponding to the aniline concentration employed in the preparation of PANI. Note that the containers need to be capped during the interfacial film formation in order to prevent rapid water evaporation, and obtain uniform and smooth thin films. The resultant films were released from the Petri dish by submerging the Petri dish in a water reservoir. The released PANI films were readily transferred onto various substrates for further studies.Fabrication of PANI thin-film humidity sensor
A released PANI thin film was transferred onto interdigital gold electrodes (100 nm in thickness and 10 μm in gap) sputter-coated on a PET sheet. The size of as-prepared interfacial PANI film was adjusted to the area of the gold electrode (2 × 2 mm2) using a cutter. The as-established device was used to determine the moisture response after drying in a fume cupboard at RT for 8 h.Measurements
UV-visible absorption spectra of PANI films on quartz substrate were recorded on a spectrometer (Perkin-Elmer, Lambda 40). Infrared spectra were recorded using an Agilent 640-IR FT-IR spectrometer with KBr method. Field emission scanning electron microscopy (FE-SEM) was performed using a JSM-7001F SEM microscope at an acceleration voltage of 10 kV. The samples were sputter-coated with platinum (Pt) for enhanced conductivity prior to SEM measurement. Electrical properties were recorded by Keithley 2400 SourceMeter. The humidity sensing analysis was carried out by monitoring the conductance of the as-fabricated sensor using a two-electrode setup. The tests under static stimuli were carried out in a sealed round bottomed flasks containing CaCl2 anhydrous for 0% RH, MgCl2 saturated solution (SS) for 32% RH, Mg(NO3)2 SS for 51% RH, NaCl SS for 75% RH, K2CrO4 SS for 86% RH,35 and deionized water for 100% RH. The sample was stored in a closed chamber with constant humidity at 20 °C for 2 h to equilibrate water of the hydration prior to each measurement of the steady-state conductance. Resistance readings were recorded for every 10 s and totally five readings were taken for each measurement. For dynamic tests, the whole sensor was placed inside a small chamber which was connected with two gas flows (i.e., wet and dry nitrogen flows in a rate of 5 ml s−1) through a 3-port ball valve, which was rapidly switchable between two gas flows. To obtain the wet nitrogen flow (a moisture/nitrogen mix flow), the nitrogen flow pasted through a flask containing water before reaching the chamber.Results and discussion
Polyaniline thin films formed at air–water interface
The polymerization of aniline by the oxidation (using ammonium persulfate, APS) in deionized water was schematically illustrated in Fig. 1a. The polymerization solution was initially colorless, but gradually turned dark during maintaining it under static condition over 24 h along with giving rise to the formation of a well-defined thin PANI film at air–water interface (Fig. 1b). The formation of the interfacial PANI film was attributed to the hydrophobic nature and favorable alignment of insoluble PANI aggregates, which was driven by solvent evaporation and the mass transports.28,30,32 The film edge was adhered to the dish wall according to the adhesive forces between PANI and the substrate surfaces, which was observed from in situ polymerization of aniline on solid substrates, as reported previously.25 The resultant films were carefully detached and released from the Petri dish by submerging the Petri dish in an open water reservoir. The PANI film was detached from the Petri dish, and floated on the surface of the water reservoir owing to the hydrophobic nature (Fig. 1c). As shown in Fig. 1d, the FE-SEM image of the thin PANI-0.04 film indicated that the film formed at air–water interface showed the fiber-type morphology packed closely on the continuous and relatively smooth surface, which agreed well with the features of interfacial PANI films reported previously.32 The thickness of the interfacial PANI film was determined to be ∼3.2 μm based on the cross-sectional analysis using SEM. There have also been detected some defects (empty holes) formed on the PANI film surface (the red circle, Fig. 1d). Rapid water evaporation could vary the uniformity of film thickness during formation of the interfacial films in this method. The released PANI film could be readily transferred onto various types of substrates including quartz (Fig. 1d, inset), silicon, PET or glass.The effect of reactant concentration on the oxidation state of PANI in the interfacial films was investigated using UV-visible spectrometry and FTIR, as the aniline concentration was varied from 0.01 M to 0.08 M. The molar ratio of aniline and APS (1/1.25) was kept constant, as described in the standard method.36 The conductivity of PANI relies closely on its oxidation states. The protonated and 50% oxidized (half oxidized/half reduced) emeraldine base (EB) form is highly conducting, while the fully reduced leucoemeraldine base and the fully oxidized pernigraniline base are mainly insulating in nature, and the rests are in between.4 UV-visible absorption spectra of the interfacial PANI films (obtained from different aniline concentrations) showed the three characteristic absorption bands at 285–314 nm, 430 nm, and 640 nm, respectively, as shown in Fig. 2a. The increase of the overall peak intensities over the whole wavelength range suggested that the film growth rate increased, as the concentration of the reactants increased. The absorption band at 285–314 nm could be ascribed to the π–π* electron transition within para-substituted benzenoid (B) segments.37–39 The absorbance of the B segments in PANI was gradually shifted from 285 to 314 nm, as the aniline concentration increased in the formation of the interfacial PANI films. The red-shift in the absorbance of the B segments indicates that the amount of B segments in PANI seems to increase upon the increase of the reactants concentration, which could enhance aniline oxidations (the doping of PANI) by ammonium persulfate (Fig. 1a). The absorption band at 430 nm is associated with polaron–π* transitions of PANI emeraldine salt.40 The absorption band at 640 nm is due to excitation transition between the benzenoid π and the quinoid (Q) π* orbitals of PANI EB.41 The intensity ratio of the band I (285–314 nm) to the band II (640 nm) allows us to estimate the oxidation state of PANI.42 The intensity ratio of band I/band II was determined to be 1.44, 1.36 and 1.52 for PANI-0.02, PANI-0.04 and PANI-0.08 samples, respectively, indicating the interfacial PANI thin films being the EB form (band I/band II = 1.7 ± 0.4).38 The PANI-0.04 sample showed the lowest intensity ratio of band I/band II, indicating the highest oxidation state of PANI.42 Note that the band II (at 640 nm) could not be clearly identified from the UV-visible absorption spectrum of PANI-0.01 sample.
 | ||
| Fig. 2 UV-visible absorption spectra (a) and FTIR spectra (b) of PANI films prepared from various solutions with different aniline concentrations. | ||
The oxidation state of PANI in the interfacial films was also evaluated based on FTIR spectra of the interfacial PANI films (Fig. 2b). The peaks at 1620 and 1382 cm−1 are associated with the absorption of sulfate species,43 and the peak at 812 cm−1 corresponds to out-of-plane bending of C–H bond present at 1,4-position of the ring.44 The bands at 1586 and 1502 cm−1 are ascribed to the ring stretching vibration of quinoid (Q), and that of benzenoid (B), respectively. The peaks at 1304 and 1144 cm−1 are due to the stretching vibration of C–N–C, and that of B–NH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q, respectively. The area ratio of these two peaks at 1586 cm−1 and 1502 cm−1 is proportional to the population ratio of quinoid to benzenoid structure in the PANI films.45 The area ratio of Q/B was measured to be 0.7, 0.75 and 0.64 for PANI-0.02, PANI-0.04, and PANI-0.08, respectively. The result indicated that the PANI-0.04 sample showed the highest content of quinoid rings, and agreed well with that obtained from the analysis using UV-visible spectrometry.
Q, respectively. The area ratio of these two peaks at 1586 cm−1 and 1502 cm−1 is proportional to the population ratio of quinoid to benzenoid structure in the PANI films.45 The area ratio of Q/B was measured to be 0.7, 0.75 and 0.64 for PANI-0.02, PANI-0.04, and PANI-0.08, respectively. The result indicated that the PANI-0.04 sample showed the highest content of quinoid rings, and agreed well with that obtained from the analysis using UV-visible spectrometry.
Humidity sensitive properties of PANI thin film
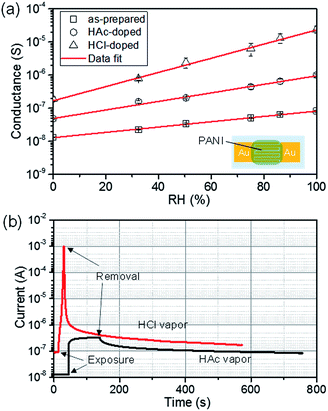
The PANI-0.04 thin film was mounted on two gold electrodes (sputter-coated on a PET sheet in 100 nm thickness and 10 μm gap) to evaluate the humidity response (Fig. 3a, inset). The sensitivity of the PANI-0.04 humidity sensor yielded the logarithmic correlation between the conductance and the relative humidity (RH) (Fig. 3a) under static condition. The conductance of the PANI-0.04 increased, as RH increased. For example, the conductance of the PANI-0.04 in dry air (0%) (1.3 × 10−8 S) increased about 5.5-fold higher than the value obtained under 100% RH (8.4 × 10−8 S).Meanwhile, we investigated the influence of doping agents on the conductance of the interfacial PANI films with an aim to improve the humidity sensitivity of the device. The doping of the PANI-0.04 film using HAC and HCl led to a significant improvement of the conductance, as shown in Fig. 3b. In addition, the conductance of the HCl-doped PANI film was higher than that of the HAc-doped film due to the higher acidity of the doping agent, as reported previously.34 The sensors based on the HAc-doped, and HCl-doped PANI-0.04 films yielded a well-defined logarithmic correlation of the conductance (G) and RH. In a simple linear regression using the equation: log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G = −A × RH + B, we found A = 0.008 and B = −7.89 (correlation coefficient R2 of 0.996) for the interfacial PANI-0.04 thin film, A = 0.013 and B = −7.31 (R2 = 0.991) for HAc-doped PANI-0.04 film, and A = 0.021 and B = −6.77 (R2 = 0.995) for HCl-doped PANI-0.04 film, respectively. Noticeably, the humidity sensitivity of the PANI-0.04 films increased by 1900% after HAc doping, and by 14
G = −A × RH + B, we found A = 0.008 and B = −7.89 (correlation coefficient R2 of 0.996) for the interfacial PANI-0.04 thin film, A = 0.013 and B = −7.31 (R2 = 0.991) for HAc-doped PANI-0.04 film, and A = 0.021 and B = −6.77 (R2 = 0.995) for HCl-doped PANI-0.04 film, respectively. Noticeably, the humidity sensitivity of the PANI-0.04 films increased by 1900% after HAc doping, and by 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000% after HCl doping, respectively. The concentration of charge carriers plays a crucial role in the conductivity of PANI.36 The very steep dependence of conductance on RH suggested a strong influence of moisture on the conductivity of PANI, especially on that of the HCl-doped PANI film.
000% after HCl doping, respectively. The concentration of charge carriers plays a crucial role in the conductivity of PANI.36 The very steep dependence of conductance on RH suggested a strong influence of moisture on the conductivity of PANI, especially on that of the HCl-doped PANI film.
The pulse-stimuli characteristics of the PANI-0.04 thin-film sensor were analyzed based on a series of current responses on dynamic switches between wet and dry nitrogen (N2) flows, as shown in Fig. 4. As the PANI-0.04 film was exposed to the moist flow (wet N2), the current of the sensor promptly jumped, and reached to a relatively stable equilibrium value within 5–7 s. Once the moist flow was switched to dry nitrogen, the current rapidly returned to the initial value within 4–7 s. The humidity sensor based on the interfacial PANI-0.04 thin film exhibited about 1.3 orders of magnitude change in the conductance with the excellent response and recovery times (defined as the time required to reaching 90% of the final equilibrium value),2 which are the critical performance parameters for advanced sensing applications. It is noteworthy that the humidity sensor based on HCl-doped PANI-0.04 film showed slightly longer response and recovery times (16–20 s and 12–15 s) than the humidity sensor based on HAc-doped PANI-0.04 films (10–13 s and 11–14 s), respectively. As a result, the response and recovery times of the humidity sensors based on the interfacial PANI films increased in the order: as-prepared PANI-0.04 < HAc-doped PANI-0.04 < HCl-doped PANI-0.04 film. The negative effect of the acid doping agents on the senor's response and recovery times would be ascribed to the enhanced hydrophilicity of the interfacial PANI film by the doping, because the sensor requires the prolonged time period to absorb higher amount of water until establishing a new equilibrium state. As shown in Fig. 5, the PANI-0.04 humidity sensors displayed good dynamic reproducible responses on moisture and exhibited excellent device characteristics. The sensor made from as-prepared PANI-0.04 particularly demonstrated the humidity sensitivity in 550% resistance change for relative humidity (RH) ranging from 0% to 100%. The result value is about twice higher than the relative sensitivity value of 291% for the humidity sensor based on electrospun PANI nanofibers.11 Furthermore, the PANI-0.04 humidity sensors fast response and recover times (5–7 s, 4–7 s) (that are two crucial factors for sensors in practice), as compared to the humidity sensors based on PANI/polyelectrolyte bilayer-structured composite (24 s and 35 s for response and recover times),46 layer-by-layer deposited poly(aniline-sulfonic acid) ultrathin film (15–27 s and 60 s),13 and electrospun PANI nanofibers (5–16 s and 2–8 s).11 It is noteworthy that the humidity sensing performance of the as-developed sensors was consistent over a month in terms of sensitivity, response and recovery times.
Conclusions
In this study, we established an approach to synthesize the PANI thin films formed at air–water interface through controlling concentration of aniline solution at a fixed ratio of aniline/APS (1/1.25). The conductivity of PANI relies closely on its oxidation states. The oxidation state of the interfacial PANI films was found to rely on the concentration of aniline in the polymerization. As confirmed using UV/visible and FTIR spectrometry, the highest oxidation state of the PANI films was yielded at an aniline concentration of 0.04 M in the interfacial polymerization of aniline, whose concentration was varied from 0.01 to 0.08 M. Furthermore, we described a sensitive and fast humidity sensor fabricated with the interfacial PANI-0.04 thin films. The humidity sensor of PANI-0.04 exhibited the sensing capability in full-range humidity with relatively high sensitivity including short response and recovery times (5–7 s and 4–7 s, respectively), as compared to other PANI-based humidity sensors reported previously.11,13,46 Noticeably, the acid doping of the interfacial PANI films using HCl or HAc could improve their conductivity, but degraded the performance of the PANI-based humidity sensors. Then, the response and recovery times increased in the order: as-prepared < HAc-doped < HCl-doped PANI-0.04 film. The use of the interfacial PANI films in the sensor system provided not only a convenient and easy tool for the electrode, but also yielded well-defined uniform and dense films, which led to the distinctive set of device performance measures including relatively high sensitivity, fast response and recovery behavior, wide work range and good repeatability, which could be required in the development of sophisticated humidity sensors.Acknowledgements
This research was supported by a research grant from Incheon National University in 2016.Notes and references
- A. Modi, N. Koratkar, E. Lass, B. Wei and P. M. Ajayan, Miniaturized gas ionization sensors using carbon nanotubes, Nature, 2003, 424(6945), 171–174 CrossRef CAS PubMed.
- Q. Kuang, C. Lao, Z. L. Wang, Z. Xie and L. Zheng, High-sensitivity humidity sensor based on a single SnO2 nanowire, J. Am. Chem. Soc., 2007, 129(19), 6070–6071 CrossRef CAS PubMed.
- K. Carr-Brion, Moisture sensors in process control, Elsevier Applied Science Publishers, London, 1986 Search PubMed.
- S. Bhadra, D. Khastgir, N. K. Singha and J. H. Lee, Progress in preparation, processing and applications of polyaniline, Prog. Polym. Sci., 2009, 34(8), 783–810 CrossRef CAS.
- G. Ćirić-Marjanović, Recent advances in polyaniline research: polymerization mechanisms, structural aspects, properties and applications, Synth. Met., 2013, 177, 1–47 CrossRef.
- Z. Xie, L. Duan, Y. Jiang, M. Xue, M. Zhang and T. Cao, Thinner is better: an ultrathin conducting oligoaniline film for gas microsensors with ultralow detection limits, Macromol. Rapid Commun., 2009, 30(18), 1589–1593 CrossRef CAS PubMed.
- D. S. Sutar, N. Padma, D. K. Aswal, S. K. Deshpande, S. K. Gupta and J. V. Yakhmi, Preparation of nanofibrous polyaniline films and their application as ammonia gas sensor, Sens. Actuators, B, 2007, 128(1), 286–292 CrossRef CAS.
- D. Li, Y. Jiang, Z. Wu, X. Chen and Y. Li, Fabrication of self-assembled polyaniline films by doping-induced deposition, Thin Solid Films, 2000, 360(1–2), 24–27 CrossRef CAS.
- J. C. Chiang and A. G. MacDiarmid, ‘Polyaniline’: protonic acid doping of the emeraldine form to the metallic regime, Synth. Met., 1986, 13(1), 193–205 CrossRef CAS.
- M. T. S. Chani, K. S. Karimov, F. A. Khalid and S. A. Moiz, Polyaniline based impedance humidity sensors, Solid State Sci., 2013, 18, 78–82 CrossRef CAS.
- Q. Lin, Y. Li and M. Yang, Polyaniline nanofiber humidity sensor prepared by electrospinning, Sens. Actuators, B, 2012, 161(1), 967–972 CrossRef CAS.
- F. W. Zeng, X. X. Liu, D. Diamond and K. T. Lau, Humidity sensors based on polyaniline nanofibres, Sens. Actuators, B, 2010, 143(2), 530–534 CrossRef CAS.
- R. Nohria, R. K. Khillan, Y. Su, R. Dikshit, Y. Lvov and K. Varahramyan, Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly, Sens. Actuators, B, 2006, 114(1), 218–222 CrossRef CAS.
- S. T. McGovern, G. M. Spinks and G. G. Wallace, Micro-humidity sensors based on a processable polyaniline blend, Sens. Actuators, B, 2005, 107(2), 657–665 CrossRef CAS.
- M. R. Gizdavic-Nikolaidis, D. R. Stanisavljev, A. J. Easteal and Z. D. Zujovic, A rapid and facile synthesis of nanofibrillar polyaniline using microwave radiation, Macromol. Rapid Commun., 2010, 31(7), 657–661 CrossRef CAS PubMed.
- J. Stejskal and I. Sapurina, Polyaniline: thin films and colloidal dispersions (IUPAC technical report), Pure Appl. Chem., 2005, 77(5), 815–826 CrossRef CAS.
- S. P. Surwade, S. R. Agnihotra, V. Dua, N. Manohar, S. Jain, S. Ammu and S. K. Manohar, Catalyst-free synthesis of oligoanilines and polyaniline nanofibers using H2O2, J. Am. Chem. Soc., 2009, 131(35), 12528–12529 CrossRef CAS PubMed.
- H. D. Tran, J. M. D'Arcy, Y. Wang, P. J. Beltramo, V. A. Strong and R. B. Kaner, The oxidation of aniline to produce “polyaniline”: a process yielding many different nanoscale structures, J. Mater. Chem., 2011, 21(11), 3534–3550 RSC.
- H. D. Tran, D. Li and R. B. Kaner, One-dimensional conducting polymer nanostructures: bulk synthesis and applications, Adv. Mater., 2009, 21(14–15), 1487–1499 CrossRef CAS.
- Z. D. Zujovic, C. Laslau, G. A. Bowmaker, P. A. Kilmartin, A. L. Webber, S. P. Brown and J. Travas-Sejdic, Role of aniline oligomeric nanosheets in the formation of polyaniline nanotubes, Macromolecules, 2010, 43(2), 662–670 CrossRef CAS.
- H. Okamoto and T. Kotaka, Effect of counter ions in electrochemical polymerization media on the structure and responses of the product polyaniline films III. Structure and properties of polyaniline films prepared via electrochemical polymerization, Polymer, 1999, 40(2), 407–417 CrossRef CAS.
- T. Cao, P. Smith and A. J. Heeger, Counter-ion induced processibility of conducting polyaniline and of conducting polyblends of polyaniline in bulk polymers, Synth. Met., 1992, 48(1), 91–97 CrossRef.
- N. Gospodinova, D. A. Ivanov, D. V. Anokhin, I. Mihai, L. Vidal, S. Brun, J. Romanova and A. Tadjer, Unprecedented route to ordered polyaniline: direct synthesis of highly crystalline fibrillar films with strong π–π stacking alignment, Macromol. Rapid Commun., 2009, 30(1), 29–33 CrossRef CAS PubMed.
- J. H. Cheung and M. F. Rubner, Fabrication of electrically conductive Langmuir–Blodgett multilayer films of polyaniline, Thin Solid Films, 1994, 244(1), 990–994 CrossRef CAS.
- J. Stejskal, I. Sapurina, J. Prokeš and J. Zemek, In situ polymerized polyaniline films, Synth. Met., 1999, 105(3), 195–202 CrossRef CAS.
- C. G. Wu and J. Y. Chen, Chemical deposition of ordered conducting polyaniline film via molecular self-assembly, Chem. Mater., 1997, 9(2), 399–402 CrossRef CAS.
- L. Tosheva, N. Gospodinova, L. Vidal, I. Mihai, M. Defaux, D. A. Ivanov and A. M. Doyle, Monoparticulate films of polyaniline, Thin Solid Films, 2009, 517(18), 5459–5463 CrossRef CAS.
- K. J. Edler and B. Yang, Formation of mesostructured thin films at the air–liquid interface, Chem. Soc. Rev., 2013, 42(9), 3765–3776 RSC.
- T. F. Wu and J. D. Hong, Dopamine–melanin nanofilms for biomimetic structural coloration, Biomacromolecules, 2015, 16(2), 660–666 CrossRef CAS PubMed.
- A. Kabalnov and H. Wennerstrom, Diffusion in evaporating solutions, Soft Matter, 2009, 5(23), 4712–4718 RSC.
- D. Chowdhury, A. Paul and A. Chattopadhyay, Macroscopic and mesoscopic patterns observed in thin films formed due to polymerization of aniline at the air–water interface, J. Colloid Interface Sci., 2003, 265(1), 70–76 CrossRef CAS PubMed.
- Z. D. Zujovic and J. B. Metson, Nanostructured aniline oxidation products: self-assembled films at the air/liquid interface, Langmuir, 2011, 27(12), 7776–7782 CrossRef CAS PubMed.
- M. V. Kulkarni, A. K. Viswanath, R. Marimuthu and T. Seth, Synthesis and characterization of polyaniline doped with organic acids, J. Polym. Sci., Part A: Polym. Chem., 2004, 42(8), 2043–2049 CrossRef CAS.
- M. Trchová and J. Stejskal, Polyaniline: the infrared spectroscopy of conducting polymer nanotubes (IUPAC technical report), Pure Appl. Chem., 2011, 83(10), 1803 CrossRef.
- J. F. Young, Humidity control in the laboratory using salt solutions—a review, J. Appl. Chem., 1967, 17(9), 241–245 CrossRef CAS.
- J. Stejskal and R. G. Gilbert, Polyaniline. Preparation of a conducting polymer (IUPAC technical report), Pure Appl. Chem., 2002, 74(5), 857–867 CrossRef CAS.
- J. Stejskal, P. Kratochvíl and N. Radhakrishnan, Polyaniline dispersions 2. UV-vis absorption spectra, Synth. Met., 1993, 61(3), 225–231 CrossRef CAS.
- Y. Wang and X. Jing, Effect of solution concentration on the UV-vis spectroscopy measured oxidation state of polyaniline base, Polym. Test., 2005, 24(2), 153–156 CrossRef CAS.
- J. Stejskal and M. Trchová, Aniline oligomers versus polyaniline, Polym. Int., 2012, 61(2), 240–251 CrossRef CAS.
- R. Sainz, W. R. Small, N. A. Young, C. Vallés, A. M. Benito, W. K. Maser and M. In het Panhuis, Synthesis and properties of optically active polyaniline carbon nanotube composites, Macromolecules, 2006, 39(21), 7324–7332 CrossRef CAS.
- C. C. Han, M. Y. Bai, K. F. Yang, Y. S. Lee and C. W. Lin, A novel method for making highly dispersible conducting polymer and concentric graphitic carbon nano-spheres based on an undoped and functionalized polyaniline, J. Mater. Chem., 2008, 18(33), 3918–3925 RSC.
- Y. Wei, K. F. Hsueh and G. W. Jang, A study of leucoemeraldine and effect of redox reactions on molecular weight of chemically prepared polyaniline, Macromolecules, 1994, 27(2), 518–525 CrossRef CAS.
- F. A. Miller and C. H. Wilkins, Infrared spectra and characteristic frequencies of inorganic ions, Anal. Chem., 1952, 24(8), 1253–1294 CrossRef CAS.
- M. Rahaman, T. K. Chaki and D. Khastgir, Polyaniline, ethylene vinyl acetate semi-conductive composites as pressure sensitive sensor, J. Appl. Polym. Sci., 2013, 128(1), 161–168 CrossRef CAS.
- S. Bhadra, S. Chattopadhyay, N. K. Singha and D. Khastgir, Improvement of conductivity of electrochemically synthesized polyaniline, J. Appl. Polym. Sci., 2008, 108(1), 57–64 CrossRef CAS.
- Y. Li, K. Fan, H. Ban and M. Yang, Bilayer-structured composite sensor based on polyaniline and polyelectrolyte for sensitive detection of low humidity, Synth. Met., 2015, 199, 51–57 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |