The gorgeous transformation of paper: from cellulose paper to inorganic paper to 2D paper materials with multifunctional properties
Xin
Dai
a and
Zhiguang
Guo
 *ab
*ab
aMinistry of Education Key Laboratory for the Green Preparation and Application of Functional Materials, Hubei University, Wuhan 430062, People's Republic of China
bState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, People's Republic of China. E-mail: zguo@licp.cas.cn; Fax: +86-931-8277088; Tel: +86-931-4968105
First published on 22nd November 2021
Abstract
Cellulose paper derived from plants is used for daily writing and recording and has historically played an important role in promoting cultural dissemination and inheritance. However, it has attracted increasing attention in recent years, serving as a flexible platform or substrate for environment and energy applications, which is attributed to its many inherent advantages such as biocompatibility, biodegradability, sustainability, low cost, and great capability to be modified. With the development of various industries, especially the flexible electronics industry, the demand for two-dimensional (2D) paper-like materials have increased. Several types of inorganic paper are manufactured and have been widely used in many fields. Among them, hydroxyapatite (HAP)-based and carbon-based inorganic paper exhibit unique advantages and have been well applied in certain fields. In addition, there are many composite papers assembled by several raw materials (cellulose fibers, HAP, and carbon), exhibiting excellent characteristics. Cellulose paper is no longer limited to traditional writing applications and has great potential to be used in many aspects, achieving a stunning change in its role. The emergence of inorganic paper and hybrid paper has further demonstrated the application prospects of paper-like two-dimensional materials. Herein, initially, we present the characteristics of three types of paper and composite paper, and then summarize their typical applications in the environment and energy fields.
1. Introduction
Tracing the history of paper development, especially the improved papermaking by Cai Lun in the Eastern Han Dynasty (also known as “Caihou paper”), is a revolution in writing materials. As an excellent carrier, cellulose paper derived from plants has been used for writing and recording, which has greatly promoted the spread and inheritance of culture and facilitated the development of civilization. However, it has attracted increasing attention in recent years, serving as a flexible platform or substrate for environment and energy applications, which is attributed to its many inherent advantages such as biocompatibility, biodegradability, sustainability, low cost, and great capability to be modified.1 The considerable modification ability of cellulose paper is mainly attributed to abundant hydroxyl groups on its surface for interactions with functional groups, porous structures for the immobilization of active materials, and large surface-to-volume ratio for the sufficient storage of reagents.2 In addition to being a flexible substrate material for supporting active layers, cellulose paper can also be used as an active material,3–7 which can be embodied in flexible electronics based on cellulose paper. Therefore, cellulose paper plays increasingly important roles that cannot be ignored in multiple fields. However, based on the natural properties of cellulose paper, it exhibits some disadvantages. For example, the hydrophilicity and high flammability of cellulose paper make it easy to absorb moisture in humid environment and be damaged at high temperature, respectively. On the one hand, researchers have proposed superhydrophobic paper and even superamphiphobic paper and explored flame-retardant cellulose paper to overcome the abovementioned natural shortcomings for expanded applications. On the other hand, these shortcomings can be exploited. For instance, cellulose paper with a hydrophilic nature can be employed as a substrate material in solar-driven water evaporators, which can quickly transport water to the upper layer for receiving heat energy.8 Taking advantage of its hygroscopic property, some researchers designed and fabricated humidity sensors.3,4In recent years, the emerging “paper-like” inorganic materials, including carbon-based paper such as graphene paper9 and graphene oxide paper,10 hydroxyapatite (HAP)-based paper,11 Si-based paper12 and other inorganic paper,13,14 have been extensively studied for their potential applications based on their characteristics. For example, HAP-based paper possesses a series of merits such as good biocompatibility, flexibility, flame resistance, and thermal insulating properties,15 providing potential opportunities for multifunctional applications in flame-retardant paper-making, fire alarms, photothermal paper-making, etc. Recently, the carbon-based composite paper containing CNT, graphene, and graphene oxide also showed promising applications, especially in flexible energy-storage devices16 and smart actuators.17 In brief, the manufacture and functional application of inorganic paper have added new vitality to the development of two-dimensional paper, and also opened new pathways for some areas that require flexible two-dimensional materials. Herein, we mainly summarize the representative applications of carbon-based and HAP-based inorganic paper in the energy and environmental fields. Furthermore, the development trends and advanced progress of paper have indicated that composite paper composed of two types of raw materials can meet the needs of various applications. Inspired by the novel tendency in paper-making, we focus on the multifunctional applications of paper, which not only can build on the advantages and characteristics of cellulose paper and inorganic paper, but also reveal the trend of paper manufacturing towards composite and diverse directions.
2. Cellulose-based paper
Cellulose, which is abundant in nature, is an important biopolymer principally found in the cell wall of higher plants, consisting of repeating β-D-anhydroglucopyranose units (AGUs).18 The organization of cellulose chains results in the formation of microfibrils, which are grouped together to form cellulose fibers (Fig. 1a) containing both crystalline and amorphous regions.19 The interchain and intrachain hydrogen bonding endow stability to the cellulose structure and lead to high axial stiffness in the cellulose fibrils, and thus crystalline cellulose possesses a higher axial elastic modulus (110–220 GPa) than Kevlar (124–130 GPa).20 The abundant hydroxyl groups in each basic unit make cellulose hydrophilic, also making it easy to bond with other functional materials.21 Cellulose paper is a flexible sheet made by pressing together the cellulose slurry suspension mainly from wood and drying it. Its microstructure is composed of randomly interconnected cellulose fibers.22 Papermaking is regarded as one of the four great inventions in China and its original and most traditional function is to write, record and print. Benefiting from its many inherent advantages (biocompatibility, renewable sources, mechanical flexibility, hydrophobicity, and 3D hierarchical architectures), cellulose-based paper can be used as a flexible substrate material or even an active ingredient, and is superior to conventional glass and plastic substrates in many aspects.22 Therefore, cellulose paper has moved from a single function to a multi-functional material that can be chosen as an available candidate in multiple fields. However, cellulose paper also has some shortcomings, such as moisture absorption due to its hydrophilicity and microbial corrosion due to its easy degradation. This part aims to present the modification technologies driven by the drawbacks of cellulose-based paper and several typically multifunctional applications based functionalized papers.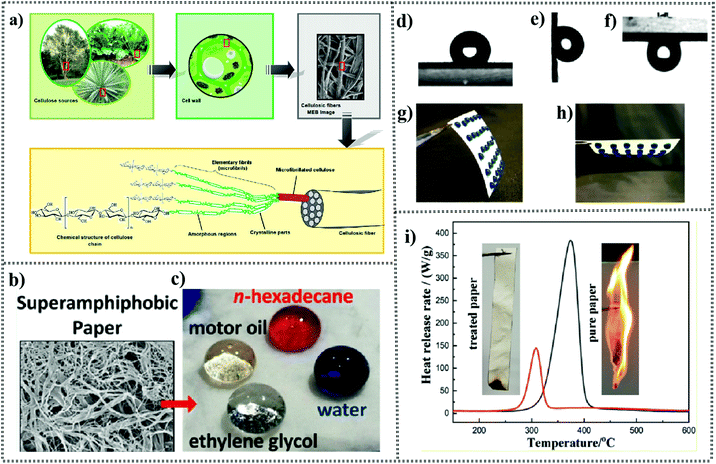 | ||
| Fig. 1 (a) Representation of a cellulose fiber showing its organization. Reproduced from ref. 44 with permission from Elsevier, copyright [2012]. (b) High-resolution SEM images of 10HW hand-sheets processed using sec-butanol before etching. (c) Droplets of four test fluids (water (dyed blue), ethylene glycol, motor oil and n-hexadecane (dyed red)) exhibit a spherical shape. Reproduced from ref. 38 with permission from the American Chemical Society, copyright [2013]. Static WCA photos (3 μL) of “1cy-ALD + heat” chromatography paper (d) 0° tilted WCA photo and (e) 90° and (f) 180° tilted WCA photos (“sticks” to paper substrate). (g and h) Photographs of an array of 3 μL drops (blue food dye was added to enhance contrast), stored on the “1cy-ALD + heat”-treated paper substrate. Reproduced from ref. 23 with permission from the American Chemical Society, copyright [2021]. (i) Heat release rate curves of pure paper sample (black line) and treated paper sample (red line). Reproduced from ref. 45 with permission from the American Chemical Society, copyright [2020]. | ||
2.1 Modifications inspired by the “shortcomings” of cellulose-based paper
Cellulose-based paper, showing some advantages of high flexibility, good mechanical properties, biocompatibility, biodegradability, and renewability, is regarded as a promising material and has been widely used in many aspects of daily life, such as packaging, decorating, cleaning, reading, and writing.1,2 However, cellulose-based paper is hygroscopic, has low microbial resistance and vulnerability to rotting, and flammable, which limit its applications especially in some severe environments such as humid conditions and high thermal state. Thus, to broaden the applications of cellulose paper, the functionalized cellulose paper obtained using multitudinous technologies possesses more “anti-natural” properties such as superhydrophobicity and even superamphiphobicity, flame-retardance, anti-antibacterial ability, and excellent mechanical durability.For example, Khanjani et al.27 prepared fluorinated cellulose nanocrystals with a nano-spherical structure via an esterification reaction with fluorine-containing reagents, followed by spin-coating the surface of cellulose paper with the resultant superhydrophobic-treated nanocrystals to obtain superhydrophobic paper. In this work, taking advantage of its hydrophilicity and dispersibility, cellulose was easily functionalized via the substitution of its hydroxyl groups with functional groups, which can be employed to construct a rough surface on paper, leading to superhydrophobic all-cellulose paper. The manufacturing processes are simple, low cost, accessible, and can yield a uniform superhydrophobic surface. Accordingly, Zhang et al.29 sprayed a suspension containing α-cellulose 10-undecylenoyl ester (CUE) on the surface of the paper to obtain superhydrophobic cellulose paper. The resultant CUE was derived by attaching 10-undecylenoyl chloride to α-cellulose. In addition, Li and co-workers33 produced food-safe superhydrophobic cellulose paper via layer-by-layer (LbL) assembly and subsequent hydrophobization treatment with carnauba wax. The polymer–nanoclay hybrid multilayers used for assembly on the paper consisted of quadlayers of carrageenan (CR)/chitosan (CS)/montmorillonite (MMT)/CS. Each layer of polymer–nanoclay hybrid multilayer could be successfully grown on the surface of cellulose paper due to the complementary opposite charges between layers. In this case, the surface roughness-mean-square (RMS) is related to the number of quadlayers for superhydrophobic cellulose paper. Moreover, graft polymerization is another efficient method to construct superhydrophobic paper for meeting practical applications.34 For example, Wu et al.34 presented a fluorine-free method to fabricate superhydrophobic and superoleophilic paper, which was applied for oil/water separation, using surface grafting of poly(perfluorooctylethyl methacrylate) (PFOEMA) via atom transfer radical polymerization (ATRP). This superwetting cellulose paper obtained via polymerization displayed a typical characteristic, where the morphology and wettability of cellulose-grafted-PFOEMA depends on the graft ratio (DG) within certain limits. The functional paper exhibited excellently chemical resistance toward a wide range of pH solution in the range of 1 to 12, which is attributed to the covalent bond of the polymer with the filter paper.
In the exploration of superhydrophobic paper, many efficient strategies have also been developed for its microstructural modification. For example, Jiang et al.37 fabricated superamphiphobic paper surfaces via the two-step combination of oxygen plasma etching and vapor deposition. The hierarchical structure generated by oxygen plasma etching not only created nanoscale roughness on the cellulose surface but also provided good adhesion between the deposited film with low surface energy and the substrate. A similar plasma etching method was also reported by Li et al.38 in 2013 for achieving superamphiphobic paper (Fig. 1b and c). Moreover, Wang and co-workers39 fabricated superhydrophobic filter paper by decorating uniform ZnO microclusters on its surface, followed by chemical modification with stearic acid. The micro/nanoscale hierarchical roughness appeared to vary based on the number of ZnO microcluster deposition cycles. In addition, by performing binary silanization with short (methyltrichlorosilane, MTS) and long (octadecyltrichlorosilane, OTS) organosilanes, Zhang et al.40 constructed superhydrophobic coatings with uniform nanostructures on several types of filter paper with different pore sizes and thicknesses. The formed nanospheres on the surface were mainly developed because of the rapid reaction between MTS molecules with the cellulose fibers, while OTS terminated the reaction and reduced the surface energy.40 In this work, they found that the thicknesses of the filter paper can dictate the “depth” of the micro/nanostructure, which in turn affect the “Cassie–Baxter state” upon in contact with water droplets. Actually, we should pay attention to the switching in the contact mode between water droplets and cellulose substrates for superhydrophobic paper.41 Recently, Li et al. reported a sticky superhydrophobic cellulosic paper with thermally stimulated wettability transformations using a combination of one-cycle atomic layer deposition (ALD) and atmospheric heating.23 The modification of the cellulose paper surface with one cycle of Al2O3 ALD provided a good environment for absorbing hydrophobic carbon upon heating.23 The abrupt transition in wettability between hydrophilicity to superhydrophobicity, which can be feasibly described by the Cassie–Wenzel and Wenzel behavior, is attributed to the adsorbed hydrophobic adventitious carbon species. The “ALD + heat”-treated paper possessed a high advancing contact angle (≥150°) and low receding contact angle (∼10°), resulting in large contact angle hysteresis (≥140°). Due to the large hysteresis, the droplets remained “pinned” to the surface even when the tilt angle reached 180° (Fig. 1d–h), which is called “sticky superhydrophobic”.23 The superwetting properties of cellulosic paper broaden its potential applications, for example, oil/water separation and microfluidics.40,42,43 In the following sections, we mainly introduce the applications of superwetting paper in oil/water separation.
The anti-antibacterial ability of superwetting cellulose paper after modification using various technologies have attracted increasing attention from researchers for broadening the applications of cellulose paper. A promising finding was that biomimetic antibacterial surfaces inspired by nature, such as cicada wings and gecko skin, need to possess nano- and microscale structures.46 Therefore, it can be assumed that superwetting materials with nano- and microscale structures may possess some antibacterial properties. For example, Baidya et al.31 fabricated a superhydrophobic paper with outstanding resistance toward bacterial and fungal growth by spray-coating waterborne fluorinated cellulose nanofiber on the surface of paper. In addition, Ahmad reviewed cellulose as support materials for antibacterial agents, and noted that cellulose has a highly reactive ability with host nano-antibacterial agents due to its abundant readily modifiable hydroxyl groups along a chain.47 In the work by Islam and co-workers, they introduced silver nanoparticles with antibacterial activity on the surface of dopamine-modified cellulose paper, resulting in the effective prevention of annexation and proliferation of microbes.48 The synthesized antimicrobial paper exhibited good antibacterial activities against virulent Gram-positive bacteria and Gram-negative bacteria, as well as some extremely strong fungal and plant pathogens. Silver possesses the advantages of broad-spectrum antimicrobial activity and high toxicity,48 making it suitable for the modification of paper and fabrication of antimicrobial cellulose paper. Amini et al.49 and Bergamonti et al.50 introduced silver into cellulose for paper, resulting in antimicrobial activity. Furthermore, Tyagi et al.51 reported the fabrication of tissue paper with satisfactory antimicrobial activity by spray-coating a hydrophobic chitosan (Ch) and cellulose nanocrystal (CNC) composite, followed by plasma treatment to further improve the antimicrobial activity of the coating. In this work, it is worth noting that the CNCs with rigid and narrow morphology played a key role in the antibacterial coating because they caused damage to the microbial cell membranes. Furthermore, by coating an acetylated cellulose solution containing the natural antibacterial agent cinnamaldehyde (CIN), Zhang et al. reported the fabrication of high-barrier, strong, and antibacterial kraft paper for green packaging.52
For example, Zhang et al.55 fabricated remarkably fire-retardant cellulose paper by adding a chitin nanofibril-based flame retardant to paper pulp. The primary reasons why chitin was chosen as a flame retardant agent are due to two main aspects. Firstly, chitin nanofibrils can be modified with P2O5 because they are rich in nitrogen and hydroxyl, and secondly is their ability to improve the tensile strength of cellulose paper. In another work by the same group, a cellulose nanofibril-based flame retardant was prepared by using a mechanochemical approach to obtain phosphorylated cellulose, followed by treatment with melamine.45 Melamine, a reagent rich in nitrogen, tends to release ammonia, diluting oxygen to retard combustion. Compared with the untreated paper, the paper loaded with 30 wt% flame retardant exhibited a reduced peak heat release rate (PHRR) by about 62.8%, its total heat release (THR) decreased from 12.5 kJ g−1 to 3.4 kJ g−1, and the percentage of oxygen required for burning the material continuously in the atmosphere (LOI) increased to 31%.45 The cellulose release rate of the paper was reduced when it was loaded with 30 wt% flame retardant, as shown in Fig. 1i. The results indicated that the paper with added flame retardant possessed excellent fire-retardant properties. Utilizing phosphorylated cellulose as a flame retardant, Chen et al.56 successfully synthesized transparent, anti-dripping and halogen-free cellulose-based flame-retardant coatings to protect for various materials such as paper. DOPO–cellulose acrylate (DCA) was prepared by modifying the cellulose chain with acrylate groups and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO). The obtained DCA with excellent solubility can easily form a three-dimensional cross-linked network on paper through a solution casting and ultraviolet irradiation process. In this flame-retardant system, the DOPO group can significantly reduce the heat release rate and total heat release and promote the formation of dense and continuous carbon, and the formed cross-linked network can inhibit melt dripping.56 In addition, LbL self-assembly is an efficient technique to fabricate flame retardant coatings. For example, Pan and co-workers57 proposed intumescent flame retardant (IFR) coatings on the surface of paper fiber by using phytic acid as a negatively charged electrolyte and a mixture of polyethylenimine (PEI) and melamine as positively charged electrolytes via the LbL self-assembly method for improving the char residue of paper. Then, zinc ions were introduced into the coating through strong coordination bonds with PEI and phytic acid for enhancing the Tmax and char residue in a nitrogen atmosphere.57
2.2 Cellulose-based paper as a substrate for multifunctional environmental applications
In this section, our aim is not to exhaust the applications of cellulose paper in the environmental field, but based on the advantages of cellulose paper, attempt to demonstrate it as a promising substrate through several typical applications. For example, the special wetting surface of cellulose paper and its many intrinsic advantages (e.g., chemical stability, low cost, biocompatibility, biodegradability and availability) make it an effective membrane material for oil–water separation or oil–water emulsion separation. Due to its microporous structure and excellent hydrophilicity, cellulose paper is used as a substrate for solar evaporators and flexible sensors. Here, we introduce several typical energy/environmental applications of cellulose paper in detail, including oil/water separation or oil/water emulsion separation, water desalination and water purification, humidity and strain/pressure sensors, and electromagnetic interference (EMI) shielding.For oil-removing surfaces, modified paper61 with superhydrophobicity and superoleophilicity was fabricated via layer-by-layer assembly, displaying efficient separation performances for stratified oil–water mixtures and water-in-oil emulsions. It is worth discussing the decline in the permeate flux and the shortening of service life to a great extent caused by the contamination of oil–water separation surfaces due to the adhesion of pathogenic microorganisms.61 Therefore, it is necessary to evaluate the anti-bacterial adhesion capability of the resultant paper in its oil/water separation performance. The experimental results of antibacterial adhesion indicated that the acquired superwetting paper could effectively suppress antibacterial adhesion compared with the original paper. Li et al.62 utilized the hydrosilylation reaction to fabricate a superhydrophobic and superoleophilic poly-(dimethylsiloxane)-co-polymethylhydrosiloxane (PDMS-co-PMHS) coating with a hierarchical micro-nanostructure and low surface energy on various porous materials for oil/water separation. When the coating was used on a filter paper substrate, the functionalized paper exhibited a satisfactory separating effect for a series of immiscible oil/water mixtures.
In 2011, for the first time, Jiang et al.63 fabricated a superhydrophilic and underwater superoleophobic surface, which displayed excellent separation performances for oil–water mixtures due to the formation of a hydration layer for protecting the surface from oil droplet contamination. Subsequently, this separation model has become effective technology for the separation of oil–water mixtures. For example, Xi et al.64 reported all-cellulose composite papers with underwater superoleophobicity for the separation of oil-in-water emulsions. Bacterial cellulose (BC) was employed as the barrier layer, the pore structure of which could be controlled though acidification and solvent exchange. It was found that efficient separation of oil–water emulsion is difficult because the diameter (d) of the dispersed phase is too small to achieve separation.65 In this separation system, the denser BC barrier layer with micron-sized pore structure distinctly improved the separation efficiency, resulting in separation efficiencies higher than 99% when the BC concentration of 0.0875 wt% and 0.1 wt% were dispersed in the alcohol–water solvent. Similarly, Ao et al.66 constructed all-cellulose composite membranes by coating a cellulose hydrogel layer on filter paper for the separation of oil/water emulsions. If “all-cellulose”-based composite papers can present the prospect for designing eco-friendly materials to explore applications in the oil/water separation field, recycling office waste paper (WP) will be another way to achieve the sustainable and green separation of oil/water mixtures. Li et al.67 found the WP with superlipophilic and superhydrophilic properties in air and superoleophobic properties underwater has the potential to separate oils from oil–water mixtures. The separation efficiency of WP for different types of oil is over 99%, and still remains above 98.9% after 30 cycles. It showed chemical stability in corrosive liquids and maintained high separation efficiency. In addition, Huang et al.68 modified tunicate cellulose nanocrystals (TCNCs) on filter paper by utilizing physical and chemical interactions, resulting in filter papers with superhydrophilicity/underwater superoleophobicity for the efficient separation of various oil/water mixtures and emulsions. The chemically TCNC modified filter papers could readily separate a hexane/water mixture and absorb methylene blue from water (Fig. 2c). Through both physical and chemical strategies, the separation efficiencies of the functionalized filter papers were more than 97% for various oil/water mixtures, and the stable cycling performance of the TCNC-modified filter paper was demonstrated for the separation of a hexane/water mixture. Moreover, Xu et al.69 proposed a cellulose-based filter paper-polyvinyl alcohol (cellulose-PVA) membrane, which could efficiently separate various types of surfactant-stabilized and different oil droplet-sized oil-in-water emulsions with 98.75% efficiency. Yang et al.70 proposed cellulose filter paper with superhydrophilicity/underwater superoleophobicity for use as an acid–alkali–salt resistant cellulose membrane for oil/water separation.
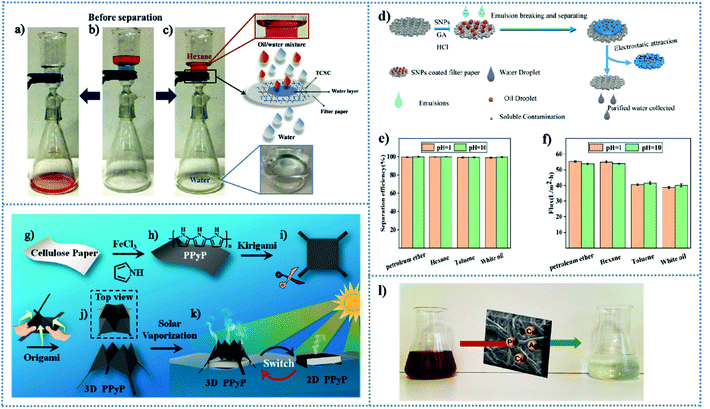 | ||
| Fig. 2 Separation performance of TCNC-modified filter papers towards oil/water mixtures. (a–c) Photographs of oil/water mixture before (b) and after separation by pristine filter paper (a) and chemically TCNC modified filter paper (c). Reproduced from ref. 68 with permission from Elsevier, copyright [2019]. (d) Fabrication process of SNP-coated filter paper and the process of oil in water emulsion separation and water absorption. (e and f) Oil/water separation performances of the SNP-modified filter paper. (e) Separation efficiency for emulsions. (f) Separation flux for hexane in water emulsions. Reproduced from ref. 71 with permission from Elsevier, copyright [2020]. (g–k) Schematic illustration of the procedure for the preparation of chemically functionalized cellulose papers with switchable 2D/3D structures. (g and h) Strategy for the fabrication of PPyP. (i) Desired 2D shape by kirigami technique. (j) 3D pyramid-like structure prepared via a kid origami game called cootie catcher. (k) Water-assisted switch of 2D and 3D PPyP. Reproduced from ref. 77 with permission from the American Chemical Society, copyright [2019]. (l) Modified cellulose paper by covalent grafting of the EDTA backbone for heavy metal remediation. Reproduced from ref. 78 with permission from the American Chemical Society, copyright [2017]. | ||
Furthermore, smart papers with pH-responsive reversible-wettability have been reported and applied in oil/water separation. Zhang et al.71 utilized pH-responsive starch-based nanoparticles (SNPs) to modify filter paper via spray-coating and crosslinking reaction for pH-sensitive controlled oil/water separation and water purification (Fig. 2d). The resultant filter paper wetted by strong acid (pH = 1) or basic water (pH = 10) showed underwater superoleophobicity, where it exhibited high efficiencies for the separation of various types of oil-in-water emulsions (Fig. 2e) and different separation fluxes (Fig. 2f). However, when the SNP-modified filter paper was wetted by weak acidic conditions (pH = 5), it failed to separate oil-in-water emulsions. The wettability transformation property is reversible at different pH environments and is important for controllable oil/water separation with a variation in the pH of emulsions. Again, Cheng et al.72 also constructed two cellulose-based papers with opposite pH-responsive reversible-wettability by grafting acrylic acid (AA) and acrylamide (AM), respectively, for controllable oil/water separation. The cellulose-g-PAA paper showed hydrophilicity/oleophobicity and hydrophobicity/oleophilicity at pH = 9 and pH = 1, respectively, and cellulose-g-PAM paper exhibited opposite properties. The separation efficiency of the two papers at both pH = 9 and pH = 1 was over 97.6%, indicating the promising applications of pH-responsive cellulose materials for oil–water separation and alleviating oil pollution.72 Finally, it is worth noting that Janus cellulose membranes73,74 with asymmetric wettability have received increasing attention for highly efficient emulsion separation in recent years.
Different from the conventional water evaporation process, where the bulk water needs to be heated, bio-inspired solar evaporators floating on the air–water interface can absorb solar light and convert it into thermal energy, which is only used to heat the extremely thin surface water layer and turn it into steam, while the bulk liquid temperature stays low, reducing the energy consumption.79 To the best of our knowledge, the reasons why cellulose-based paper has opportunities to become a supporting platform for solar absorbers for the construction solar evaporators can ascribe to the following points: (1) cellulose paper with high capillary power due to its microporous structure and excellent hydrophilicity can continuously pump water from the bulk to the interface, supporting the successful conversion of water to steam.8,79 (2) Due to its low thermal conductivity and excellent thermal insulation, cellulose paper a as floater reduces the heat loss to the environment.80 (3) The abundant functional groups on cellulose paper allow its modification with various photothermal materials for the construction of bilayer structures.20 Naturally, the top photothermal material needs to have two main properties, i.e., the ability to capture solar light and transform energy from light to heat.
At present, bilayer structural photothermal materials composed of a top photothermal layer and bottom fiber paper as the support layer have been reported. Wang et al.79 fabricated reusable plasmonic membranes (PMs) for solar steam generation by depositing Au nanoparticles (NPs) on microporous filter paper decorated with poly(diallyl dimethyl ammonium chloride) (PDDA). Au NPs as the main absorbers of solar light were used to achieve light-to-heat conversion through the surface plasmonic effect.81 Under 10 kW m−2 irradiation, the resulting composite paper achieved a high steam generation efficiency of 85% and the evaporation rate of 11.8 kg m−2 h−1 after irradiation for 60 min. Liu et al.82 also reported the fabrication of a filter paper-based solar evaporator by embedding Au NPs on the surface of paper. In addition, the excellent light absorption properties of Ti2O3 nanoparticles provide opportunities for their application in solar thermal conversion systems. For example, Wang et al.83 designed a solar water steam evaporator with a bilayer structure via the deposition Ti2O3 NPs on a cellulose membrane under vacuum conditions. Carbon materials are regarded as an excellent type of photothermal materials. For example, multiwalled carbon nanotubes (MWCNTs) show remarkable solar absorbance over a wide spectrum range and a high light-to-heat conversion efficiency.84 Huang et al.8 reported the preparation of a highly efficient bilayer photothermal paper by combining a top multiwalled carbon nanotube (MWCNT) layer and a bottom polyphenylene sulfide/fibrillated cellulose (PPS/FC) paper for water desalination and purification.
Moreover, it has been demonstrated that polypyrrole (PPy) has good light absorption, strong interaction with cellulose and facile preparation methods.77,85 Ni et al.77 fabricated a PPy chemically functionalized cellulose paper (PPyP) through an in situ controlled oxypolymerization reaction to construct a solar steam generator with switchable 2D/3D structure for performing photothermal water purification in diverse environments (Fig. 2g–k). The solar energy evaporation system consisted of the cellulose-based photothermal solar evaporator and a floating polystyrene (PS) foam support board with excellent thermal insulation properties. In the case that the area of the water surface was too small to accommodate the unfolding 2D structure, due to the structural advantages of the folded 3D structure, it exhibited a remarkably enhanced evaporation rate, resulting in excellent water evaporation as high as 2.99 kg m−2 h−1 under 1 sun.
At present, one of the challenges in the use of photothermal membranes for water desalination is the problem of salt scaling on their surface after a period of distillation, resulting in reduced evaporation efficiency.81 Therefore, it is of great significance to design solar evaporators with salt self-cleaning ability. For example, Xu et al.81 designed a photo-thermal membrane with superhydrophilic property and high porosity (80%) via the deposition of PPy shells onto cellulose fiber (PCF) paper to achieve salt auto-cleaning capacity. Benefitting from the PCF with high porosity, which provides an unobstructed channel for water transportation, the scaled salt spontaneously transferred back to the bulk seawater in the dark under the drive of the chemical potential difference of the salt. Therefore, the resultant evaporator could maintain a high and constant seawater evaporation flux of 1.41 kg (m2 h)−1 and did not need to be manually cleaned during the evaporation cycle of several days, enabling stable long-term solar seawater desalination.
For the removal and absorption of heavy metals, the common and untreated cellulose does not exhibit excellent performances, whereas cellulose with abundant hydroxy groups can be regarded as well-functionalized objects.86,87 Therefore, it is significant that some research is focused on the surface functionalization of cellulose for developing biodegradable and efficient materials to removal heavy metals. Taking advantage of the high hydrophilicity of cellulose paper and the strong chelating properties of EDTA for metals, d'Halluin et al.78 reported a modified cellulose paper by covalent grafting an EDTA backbone for heavy metal remediation in wastewater treatment. The resulting paper-based adsorbent could maintain effective operation with 95% absorption over a wide range of pH values ranging from 3 to 9 for a solution with less than 5 ppm of Cd(II) and Pb(II). In addition, its adsorption capacity for various heavy metals was also evaluated and the experimental results were satisfactory. As shown in Fig. 2l, the chemically modified cellulose filter paper with EDTA could efficiently remove the metal under neutral conditions. Nongbe et al.88 constructed two cellulose-based polyaminated adsorbents, ethylenediamine-grafted cellulose paper (Cell-Ed) and spermine-grafted cellulose paper (Cell-Sperm), for the removal of heavy metals. Cell-Sperm exhibited excellent adsorption capacity not only for Cu(II) and Pb(II) but also for Zn(II), Cd(II) and Fe(II). Briefly, the chemical methods for the modification of cellulose paper mentioned above can be summarized into functionalization of the hydroxyl groups of cellulose, where the introduced groups usually have strong affinities with contaminants. In the case of the application of cellulose paper as a supporting platform for water purification, there are some reports about the removal of other contaminants in water. For the removal of arsenate from drinking water, Pramanik and co-workers87 introduced 3-mercapto-propanoic acid (MPA) as a functionalization reagent to modify cellulose filter paper, resulting in an adsorbent (MPA-Cell paper). Nanoscale zero valent iron (NZVI) as a nano-adsorbent is used for water remediation, but its easy aggregation leads limits it reactivity and stability.89 Benefiting from the biocompatibility and porosity of cellulose paper, Arshadi et al.90 reported the preparation of NZVI monodispersed filter paper for the sequestration of phosphate ions from wastewater. The resulting paper displayed a remarkable phosphate removal efficiency of 99.9% for certain phosphate ion amounts and contact times and exhibited high reusability after 7 adsorption–desorption cycles.
Taking advantage of the hygroscopicity of cellulose paper, Whitesides et al.3 designed a humidity respiration sensor by converting the changes in humidity into electrical signals. The amount of water on the surface of the cellulose fibers can vary in the process of breathing out and in, resulting in a variation in the ionic conductivity of the sensor, and furthermore showed a fluctuant data stream, as shown in Fig. 3a and b. The chemical structure of the cellulose and the water molecule adsorption process and proton hopping conduction are shown in Fig. 3c and d, respectively. Pure paper is insulated at low relative humidity, but when the relative humidity is too high, water dissociation occurs on the wet cellulose fibers because the ordering of the water molecules may gradually disappear and protons will have increased freedom to move inside the condensed water through the Grotthuss mechanism, leading to the production of H+ and OH−, which is an ionic conduction mechanism.107,108 The high humidity (RH 100%) during exhalation and the large humidity change during breathing can provide an application environment for this respiratory sensor. In this case, paper not only played the role of a flexible biocompatible substrate but also acted a sensing material.109 In another example of ion conduction, Duan et al.4 fabricated a multifunctional humidity sensor via a simple pasting method, employing cellulose paper as the humidity sensing material and conductive adhesive tape as the flexible electrode. The sensor could be used in multiple humidity-related applications, such as monitoring of the breathing rate, baby diaper wetting, non-contact switching, and skin humidity.4
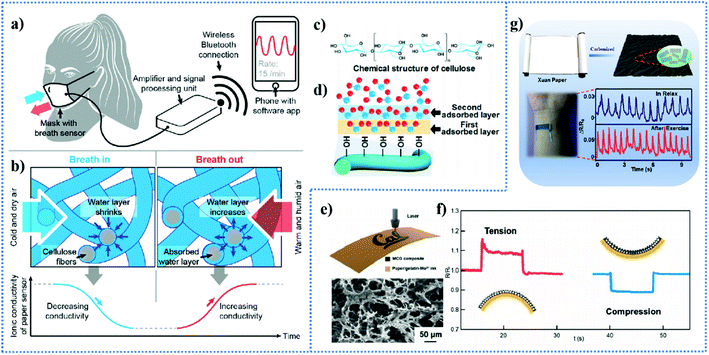 | ||
| Fig. 3 (a) Schematic illustration of the facemask for respiration monitoring with the embedded paper-based sensor and electronics. (b) Mechanism of the operation of the paper-based electrical respiration sensor. Reproduced from ref. 3 with permission from the German Chemical Society, copyright [2016]. (c) Chemical structure of cellulose. (d) Water molecule adsorption process and proton hopping conduction. Reproduced from ref. 4 with permission from the American Chemical Society, copyright [2019]. (e) Schematic diagram of the fabrication process for MCG composites on top of a paper substrate and the top view SEM micrograph for the surface morphology of the MCG composites with a porous structure and coral-shaped flakes. (f) Response and recovery time for the prototype sensor under tensile and compressive strain, respectively. Reproduced from ref. 110 with permission from Elsevier, copyright [2020]. (g) Illustration showing the structure and the carbonization process of Chinese art paper and relative resistance change induced by pulse vs. time in the relaxed state and after exercise. Reproduced from ref. 5 with permission from the American Chemical Society, copyright [2019]. | ||
The advantages of paper as a substrate include its low bending stiffness for better flexibility,111 hydrophilicity for hygroexpansion,22 and porous structure for easy modification with functional reagents,112 which have aroused the interest of researchers in developing paper-based humidity sensors with highly sensitive responses. To improve the performances and broaden the application of paper-based sensors, humidity sensors combining carbon materials and cellulose paper have been reported by many researchers. For example, Han et al.107 utilized cellulose paper as a substrate and loaded functionalized CNTs with carboxylic acid (COOH) on its surface to fabricate a humidity sensor. Different from the aforementioned full-cellulose respiratory humidity sensor, the CNT-paper sensor mainly utilizes the influence of humidity on the conductivity of CNTs to respond to changes in humidity. The resultant sensor possessed linear behavior up to a relative humidity of 75% with good repeatability and low hysteresis.107 Some researchers have developed conductive inks for flexible electronic sensors, especially carbon nanotube inks (CNT inks) have received significant attention.113 For example, Parrilla et al.113 fabricated a wearable paper-based sweat sensor by coating single-walled carbon nanotube (SWCNT) ink based on sodium dodecylbenzenesulfonate (SDBS) surfactant on the surface of filter paper. The response mechanism of the sensor to humidity can be simply explained by the change in electrical resistance in the humidification process, during which water swells the cellulose fibers, causing an increase in the distance between the carbon nanotubes wrapped by the fibers. The paper-based sensor displayed high sensitivity and reproducibility for monitoring the liquid content. In addition, Zhao et al.114 reported the fabrication of humidity sensors by depositing oxidized multi-walled carbon nanotube (o-MWCNT) ink on paper substrates. The o-MWCNTs with high oxidation levels can enhance the water adsorption capacity, and the water molecules adsorbed on the top layer of the paper can donate electrons to the o-MWCNTs, and thus the sensor shows a higher response. The optimized paper-based sensors exhibited a high response (∼33%) and excellent linearity (R2 = 0.9978) from 33% to 95% RH.114 There are some other reports on carbon material-cellulose paper humidity sensors.113,115–117
Besides the use of carbon-based materials as a sensing layer, Sahatiya et al. fabricated a multi-responsive sensor for monitoring changes in humidity and temperature, breath sensing and ethanol contamination by introducing an MoS2–Cu2S hybrid on cellulose paper via the hydrothermal method.118 Using chemical modification for improving the response speed of paper-based humidity sensors, Guan et al.119 introduced glycidyl trimethyl ammonium chloride (EPTAC) on the cellulose fibers of printing paper, resulting in a decreased in the response time to 25 s compared with that of the blank paper of 101 s. The resultant sensor exhibited multifunctional applications in respiratory monitoring, non-contact switch and skin humidity monitoring.119
Similarly, cellulose paper plays dual roles in the construction of pressure/strain sensors, which can be roughly classified as a substrate for supporting sensing materials and raw material for preparing functional sensing layers. Here, we discuss the former. To the best of our knowledge, the selection of the upper conductive materials is one of the prominent problems in the multifunctional application of flexible strain sensors in real life. Therefore, researchers are inclined to choose the combination of well-known substrates such as paper and compliant conductive materials such as carbon-based materials instead of some metals or semiconductors.120,121
Cellulose paper as a substrate of strain sensors supports various conductive sensing materials to achieve resistance changes under deformations, in which the conductive materials account for the resistive-type sensitivity.120 Zhan et al.121 reported a paper-carbon nanotube-based wearable pressure sensor by impregnating single-wall carbon nanotubes into tissue paper (SWNT/tissue paper) and assembling it with a PDMS sheet and polyimide (PI) sheet patterned with interdigitated Au electrodes. The pressure response of the sensor was achieved by the deformation of the soft tissue under external pressure to make more single-walled carbon nanotubes contact the interdigital electrodes on the paper, thereby improving the conductive pathways.121 The SWNT/tissue sensor exhibited excellent pressure response performances and was successfully used to detect human vital physiological signals, such as radial artery pulse and muscle motion. In another work reported by Long et al.,110 piezo-resistivity pressure/strain sensors were fabricated by introducing molybdenum carbide–graphene (MCG) composites with porous and stacking micro-structures on paper substrates via a direct laser writing method. The working mechanism of the pressure/strain sensors was discussed in detail (Fig. 3f). In brief, the porous and coral-shaped microstructure of the MCG composites can change the resistance caused by the breakage and formation of interconnection under mechanical deformation. When it suffers tensile strain, the micro-cracks become larger and some interconnection may be broken, resulting in an increase in resistance and decrease in conductivity. In contrast, compressive deformation leads to the opposite situation. The response time and recovery time of tensile test and compressive strain test were satisfactory, among which the response time of tensile strain test was 0.126 s and recovery time was 0.195 s, and the response time of compressive strain was 0.166 s and recovery time was 0.124 s. Besides a fast response and recovery time, the sensors exhibited high sensitivity and could detect various types of human body motions and some weak pressure signals. It is necessary to mention that the working mechanism of the pressure sensor includes resistive, capacitive, and triboelectric mechanisms.122 For resistive-type carbon-paper strain/pressure sensors, the carbon materials appearing in related reports also include reduced graphene oxide (RGO),123 graphite,124,125 carbon black (CB),120 CB/CNT25 and graphene.111 In addition to carbon materials, the conductive sensing materials used in paper-based strain/pressure sensors include MXenes126 and silver nanowires (AgNWs).127,128
The sensitivity of the traditional resistive strain sensors mainly depends on the geometric effect of the active material, while the sensitivity of flexible resistive strain sensors depends on the structural change of the active material, such as crack propagation and disconnection between the active materials.129 It has been found that the presence of 2D or 3D micro/nanostructures can effectively improve the sensitivity of strain/pressure sensors.130,131 Therefore, in addition to pursuing new materials with excellent properties for the design of sensors, the structural design of sensing materials is also very important. Inspired by the vibration-sensing organ of the scorpion and lotus leaf, Liu et al.130 designed biomimetic, superhydrophobic paper-based strain sensors for wearable and underwater applications. They designed the paper substrate into a V-shaped groove structure, where compressive loads deformed the bottom of the groove, reducing the distance between the two walls of the groove, and at the bottom of the groove, the two walls of the groove even touched. Therefore, after loading conductive nanoparticles on the predesigned structured paper, the pressure response of the sensor originated from the change in contact resistance. The resultant paper sensors treated with hydrophobicity will hopefully solve the challenge of rapid degradation of paper-based electronics upon exposure to moisture, paving the way for the development of sensing materials for underwater vibrations.130 In another typical case, Liao and co-workers131 fabricated self-waterproof crack-based resistive bending strain sensors by depositing gold NPs on the surface of the abrasive paper with peaks and valleys.
Here, we introduce paper as an active material, in other words, the resultant carbon derivatives from paper are used for the fabrication of strain/pressure sensors. Xia et al.5 proposed a carbonized Chinese art paper-based high-performance wearable strain sensor for human activity monitoring. The carbonized Chinese art paper after annealing treatment formed a good carbonized fiber-to-fiber conductive network, which was subsequently encapsulated with an elastic matrix for the preparation of the sensor. The strain sensor exhibited ultrahigh sensitivity (gauge factor (GF) of 68 during the strain of 100% and 248 in strain range of 100–120%), ultralow monitoring limit (0.01% strain), outstanding durability, stability, and fast response (<50 ms). Therefore, it was applied to detect subtle human movements, for example, pulse variation in the relaxed state and after exercise could be transformed to electrical signals with a resistance change (Fig. 3g). Similarly, Li et al.132 constructed a highly flexible piezoresistive strain sensor composed of carbon paper obtained after high-temperature carbonization of tissue paper and polydimethylsiloxane (PDMS) elastomer. Moreover, Chen et al.6 also utilized the heat-treatment method to obtain a conductive carbon fiber network from crepe cellulose paper with a corrugated structure and further encapsulated it with PDMS for the preparation of an anisotropic strain sensor. Then, this group reported another pressure sensing system7 employing carbonized crepe cellulose paper. In addition to sensors with only a special sensing property but applied in multiple situations, there are some multimodal sensors with the ability to respond to multiple stimuli. For example, by spraying a mixture of CB and RGO on a paper substrate, Liu et al.117 fabricated a flexible multimodal sensor with multi-sensor responsiveness to strain, humidity, temperature and pressure.
According to the EMI shielding mechanism, some materials with high electrical conductivity have been introduced into cellulose paper for achieving EMI shielding such as carbon nanotubes,138,139 Ag nanowires,140,141 and MXenes.135,136 Lee et al.138 reported the preparation of multi-walled carbon nanotube (MWCNT)-coated cellulose papers via the dip-coating method, showing high electromagnetic interference shielding. The shielding effectiveness (SE) is a common parameter used to quantitatively describe the shielding effect of EMWs in decibels (dB),133 where EMI shielding materials with 20 dB (99% attenuation of EMWs) can meet commercial requirements.142 The MWCNT/cellulose paper fabricated by Lee and co-workers exhibited a high SE value of ∼20.3 dB at 1 GHz, achieving ∼99.1% attenuation of electromagnetic waves. Silver nanowires, a material with high conductivity, small diameter, and high aspect ratio, have been used for the fabrication of high-performance EMI shielding materials.140,141 In another work reported by Lee in the same year, they constructed AgNW-coated cellulose papers using the same dip-coating method.140 The fabrication process of AgNW/cellulose paper is shown in Fig. 4a. The AgNW/cellulose paper showed an excellent EMI shielding performance of ∼48.6 dB at 1 GHz when the amount of AgNWs introduced was low, i.e., 0.53 vol%. It has been found that poly(vinylpyrrolidone) (PVP) can help AgNWs to coat tightly on cellulose paper via specific interactions,140 which also result in some drawbacks such as its insulting property increases the contact resistance.143
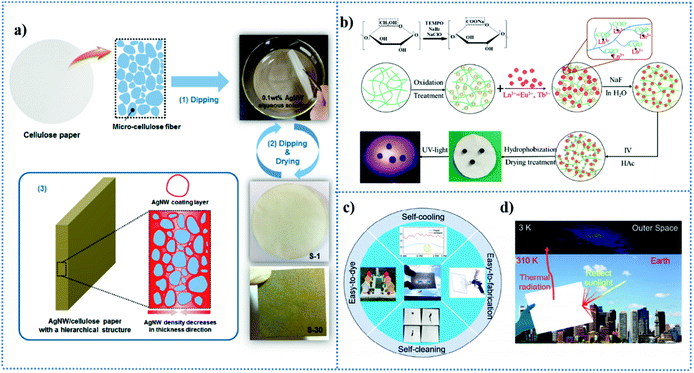 | ||
| Fig. 4 (a) Fabrication process of AgNW/cellulose paper with a hierarchical structure via the dip-coating process. Reproduced from ref. 140 with permission from the American Chemical Society, copyright [2016]. (b) Schematic for the fabrication of TOC@LaF3:Eu3+@TiO2-based superhydrophobic anticounterfeiting paper. Reproduced from ref. 146 with permission from the American Chemical Society, copyright [2020]. (c) Multifunctional paper-based composites with self-cooling and self-cleaning properties can be easily fabricated and dyed. (d) Photograph and schematic showing that the composites strongly backscatter the solar irradiance and re-emit thermal radiation, resulting in a net radiative heat loss to the outer space. Reproduced from ref. 147 with permission from the American Chemical Society, copyright [2020]. | ||
The penetration of water reduces the conductivity and EMI SE of materials. Therefore, Zhan et al.141 fabricated a superhydrophobic cellulose paper coated with AgNWs, and subsequently deposited nickel nanoparticles (Ni NPs), showing an excellent EMI SE of 88.4 dB. The addition of Ni NPs can improve the electrical conductivity and magnetic performance, leading to high absorption shielding, which is beneficial for eliminating EMI waves and avoiding secondary pollution. In some situations dominated by EMI absorption,144 shielding materials with satisfactory EMI absorption have received attention. Zhang et al.145 reported a superhydrophobic absorption-dominated electromagnetic shielding material by introducing clustered Fe3O4 nanoparticles on copper-coated cellulose paper. The clustered Fe3O4 microspheres with excellent magnetic and conductive properties can facilitate EMW effective dissipation, and multiple reflections and scattering, causing more EMW absorption between the copper-plated and magnetic layers. The material exhibited wave-absorbing-based shielding effectiveness of >50 dB (absorption: reflection ∼95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5).
5).
MXenes (Ti3C2Tx) are materials possessing a high specific surface area, superior inherent conductivity and strong EMI wave absorption, and thus applied as EMI shielding materials.144 Hu et al.135 reported a cellulose/MXene nanocomposite paper, which was prepared by dip-coating cellulose paper with an MXene suspension, followed by coating with polydimethylsiloxane (PDMS) for efficient electromagnetic interference shielding. The EMI shielding performances of the resulting cellulose/MXene paper were reflected in its high EMI SE of over 43 dB in X and Ku-band at the Ti3C2Tx loading of 1.07 vol%, and outstanding mechanical stability after 2000 bending–releasing cycles.135 In another example of the use of MXene as an EMI shielding material for paper, Li et al.136 used MXene/PPy ink and PDMS/candle soot to coat the two surfaces of paper to prepare a multifunctional and superhydrophobic cellulose composite paper. In their work, it is worth mentioning that the resulting paper not only exhibited a high EMI SE of ∼40 dB but also could be used as a hydraulic triboelectric nanogenerator (HTENG) to harvest water energy, which met the prospects and challenges of multifunctional applications of paper in the future.
Nawaz et al.148 reviewed the methods for the chemical modification of cellulose-based materials such as 2D cellulose paper into smart fluorescent materials and their multifunctional applications, including anticounterfeiting,146,149,150 chemical sensing,151–153 and gas detection.154 For example, Wang et al.146 constructed a superhydrophobic and photoluminescent cellulose-based paper for anticounterfeiting application by doping lanthanide-based nanocrystal/nanotitania in cellulose fibers. The fabrication and photoluminescent properties of the TOC@LaF3:Eu3+@TiO2-based superhydrophobic anticounterfeiting paper are shown in Fig. 4b. The as-prepared two types of paper presented different colors under 365 nm UV irradiation, which can be used in practical anticounterfeiting applications demanding hydrophobicity due to their properties of superhydrophobicity, mechanical stability and security. Wang et al.154 reported an ultrasensitive fluorescent paper-based CO2 sensor working by a fluorescence shift from teal to orange in the reversible protonation reaction of bis(4-pyridyl)-dineopentoxyl-p-phenylenedivinylene (Np-P4VB) deposited in the cellulose fibers of the paper. The fluorescent sensor exhibited a short response time of around 1 min and has the potential to be used to sense multicomponent atmospheric gases that do not contain other acidic gases. Fluorescent paper-based test strips have high sensitivity, fast response, and facile and low-cost operations, and show advantages in detecting some chemicals, especially in dealing with real-time and field assays.153,154 Cellulose-based paper has been regarded as a substrate with a large modified space for achieving multifunctional applications, which again can be reflected in the fabrication and applications of fluorescent paper.
In addition to the applications of cellulose paper mentioned above, it is worth mentioning its application in the aspect of radiative cooling. The use of cellulose paper for radiative cooling has rarely been investigated to date. In fact, paper consisting of cellulose fibers (20–50 μm in diameter), as a material with high solar reflectivity (Rsolar) of about 0.89 and an excellent infrared thermal emissivity (εIR) of 0.92, is a good candidate for passive daytime radiative cooling (PDRC).155 Tian et al.147 reported the preparation of superhydrophobic paper-based composites with self-cooling and self-cleaning properties by coating poly(tetrafluoroethylene) (PTFE) particles with extremely high reflectivity and extremely low surface energy via physical adsorption and mechanical interaction. The superhydrophobic coating endowed the paper with self-cleaning properties and could avoid the reduction of radiative cooling performances caused by contaminations such as floating dust, dirt, and soot on the surface of the paper (Fig. 4c). As shown in Fig. 4d, the resultant paper exhibited strong backscattering of the solar irradiance and re-emitted thermal radiation. The resultant self-cooling paper exhibited a subambient cooling performance of 5 °C under a solar irradiance of 834 W m−2 and a radiative cooling power of 104 W m−2 under a solar intensity of 671 W m−2.147 Moreover, the emergence of this radiative cooling paper not only can broaden the multifunctional applications of paper but also can pave the way for the development of energy-efficient and eco-friendly cooling systems.
3. Inorganic paper
In the review by Wen et al., they focused on several novel inorganic papers made from four types of raw materials (hydroxyapatite (HAP), silicon, carbon, and metal–organic frameworks (MOFs)), highlighting their design principles and new fabrication methods.14 The multifunctional applications of inorganic paper in four main aspects were mentioned, including fire-resistance and anti-bacterial property, pollutant treatment, actuators, and flexible electronics. In this part, we focus our attention on the typical applications of HAP-based and carbon-based inorganic paper based on their inherent features and advantages. The emergence of inorganic paper has broadened the application range of two-dimensional paper-like materials. Especially in the development towards multi-functional applications, hybrid composites of cellulose fibers, HAP and carbon have appeared, which make the synthesis and development of paper develop towards more diversified directions.3.1 Properties of HAP-based paper as a substrate and its multifunctional applications
Hydroxyapatite (HAP), with the general formula Ca10(PO4)6(OH)2, is the primary mineral component of bone and teeth.156 Synthetic HAP materials have some outstanding properties such as non-toxicity, biocompatibility, adsorption capacity, acid-based adjustability, ion exchange capability, thermal stability and resource recovery.156 Therefore, various synthetic HAP materials have been regarded as excellent candidates for various biomedical applications.157 In their recently reported review, Ibrahim et al.156 introduced HAP materials, ranging from their primary structural characteristics and inherent properties to their main applications in the field of environmental management such as water decontamination, air clean-up and soil treatment. HAP with various structures and morphologies was fabricated by multiple methods, which were grouped into five simple categories (dry methods, wet methods, high-temperature processes, synthesis from biogenic sources and combination procedures).158However, the structure, size, morphology and crystallinity of synthetic HAP materials strongly affect their physicochemical properties and applications.157 Noticeably, one-dimensional HAP nanowires (HAPNWs) became a material of concern, largely driven by the successful fabrication of ultralong HAPNWs, which was first reported by Zhu et al. in 2014.11 HAPNWs were fabricated by employing calcium oleate as the precursor and NaH2PO4·2H2O as the phosphorus source via the solvothermal method. The scanning electron microscopy (SEM) micrographs and transmission electron microscopy (TEM) micrograph in Fig. 5b–d show that the product obtained after solvent heat treatment is composed of HAP ultra-long nanowires with a single-crystal structure.11 The as-prepared HAPNWs had higher aspect ratios (>100) than the previously reported HAPNWs, resulting in ultralong HAPNWs with high flexibility. Furthermore, the emergence of ultralong HAPNWs makes it possible to fabricate HAP-based inorganic paper with fire-retardance, flexibility and excellent mechanical properties, and overcome the disadvantage of the high brittleness of common inorganic paper. The HAP-based paper made from ultralong HAP nanowires and Na2SiO3 as an inorganic binder possessed enhanced strength (Fig. 5a). At present, HAP-based inorganic paper has been used as printing or writing paper with excellent fire-resistance and high thermal stability, which has certain advantages compared to flammable cellulose paper. However, besides writing, it is another significant topic in the future to integrate various functional agents into pure HAP-based paper for achieving multifunctional applications,159 which has been demonstrated by the results reported in the last few years. In this section, we present the related applications of inorganic paper made using HAPNWs, not only as writing paper but also in terms of environment and energy applications.
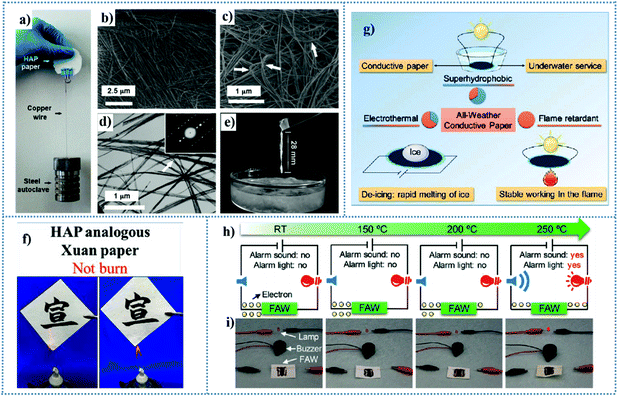 | ||
| Fig. 5 (a) Piece of HAP paper (diameter ∼5 cm, thickness 0.3 mm) made from ultralong HAP nanowires and Na2SiO3 as an inorganic binder to enhance its strength. Characterization of typical ultralong HAP nanowires (b–e). (b and c) SEM micrographs and (d) TEM micrograph. Inset of (d) is the selected-area electron diffraction (SAED) pattern of a single HAP nanowire. (e) Formation of a long fiber with a length of 28 mm from stirring a dispersion of HAP nanowires in ethanol. Reproduced from ref. 11 with permission from Wiley-VCH, copyright [2014]. (f) Resistance to fire and high temperatures of the as-prepared inorganic analogous Xuan paper. Reproduced from ref. 160 with permission from the American Chemical Society, copyright [2018]. (g) Schematic illustration of the novel design of a new type of the all-weather flexible electrically conductive paper based on ultralong hydroxyapatite nanowires with the unique combination of a superhydrophobic surface, electrothermal effect, and flame retardancy. Reproduced from ref. 161 with permission from the American Chemical Society, copyright [2017]. (h) Schematic illustration showing the changes in an alarm lamp, alarm buzzer, and electrons in an electrical circuit after thermal treatment of FAW with GO thermosensitive sensor at different temperatures for 3 min (RT: room temperature). (i) Corresponding digital images. Reproduced from ref. 162 with permission from the American Chemical Society, copyright [2018]. | ||
The combination of superhydrophobicity and fire resistance in HAP-based paper is necessary for adapting to more application environments. Chen et al.165 reported the preparation of superhydrophobic and fire-resistant layered inorganic paper via the assembly of ultralong HAPNWs and after surface modification with sodium oleate. The hierarchical structure of the microfibers formed by HAPNWs increased the roughness and sodium oleate as a grafting agent decreased the surface energy of HAPNWs, leading to a superhydrophobic inorganic paper with not only high thermal stability and excellent resistance to mechanical destruction but also good liquid repellency and self-cleaning ability. The as-prepared paper could be employed in multifunctional applications, for example, fire-shielding protection, oil/water separation, and writing and printing paper with water-proof and fire-resistant properties. Both HAP-based inorganic paper and cellulose-based paper have high biocompatibility and good flexibility, but only the former has a natural advantage of thermal stability. Cellulose paper has been regarded as a favorable substrate for constructing flexible devices, which has been strongly demonstrated in the numerous reports in the literature, as mentioned in the section on cellulose-based paper for flexible electronics. Naturally, what triggered our thinking is the potential application of inorganic paper based on ultralong HAPNWs with both superhydrophobicity and fire resistance in flexible electronic devices. Chen and co-workers161 explored and designed an all-weather flexible electrically conductive inorganic paper with flame retardancy and superhydrophobicity using ultralong HAPNWs, Ketjen black (KB), and poly-(dimethylsiloxane) (PDMS) (Fig. 5g). Firstly, the current stabilities of the paper when it encountered water droplets and immersed in water were investigated. The results indicated that whether water droplets were deposited on the surface of the paper or the paper was immersed in water, the current change in the paper was very small, varying by 0.38% in the former condition, and 3.65% for 120 s in the latter condition, respectively. Secondly, the effect of fire on the electronic conductivity of paper was also investigated. After exposure to a flame for 7 min, the resulting paper preserved its structural integrity and the electrical current flowing through the burnt paper was maintained as high as 90.60% of the original value. In addition, the paper could reach a high temperature in a short time for rapidly melting ice.
Furthermore, by utilizing the ultralong HAP-based paper with good thermal stability and high flexibility and GO with variable conductivity when exposed to flames, a fire alarm wallpaper (FAW) was constructed by integrating HAPNWs as a fire-resistant substrate and graphene oxide (GO) as a thermosensitive sensor.162 Briefly, the working mechanism can be described as the instantaneous deoxidation of the graphene oxide thermal sensor caused by the increase in temperature, which causes the electrical insulation state to quickly change to the conductive state. As shown in Fig. 5h, the GO thermosensitive sensor on the FAW was connected to an alarm lamp and an alarm buzzer, and the conductive line had no signal display until the temperature increased to 250 °C. Moreover, after modification with polydopamine, the modified GO could respond to a low thermal temperature (126.9 °C), had a rapid response time (2 s) and maintained a long alarm time in the flame (at least 5 min). In addition, Cheng et al. combined HAPNWs with flexibility and boron nitride nanosheets (BNNSs) with high in-plane thermal conductivity and good water dispersibility to obtain a flexible and fire-resistant all-inorganic composite film.166 The in-plane thermal conductivity (TC) value of the as-prepared composite films reached 6.4 W m−1 K−1 at 25 °C at the BNNSs fraction of 30 wt%, which again proves the possible development of HAP-based paper as a substrate material, especially under conditions requiring high-temperature stability.
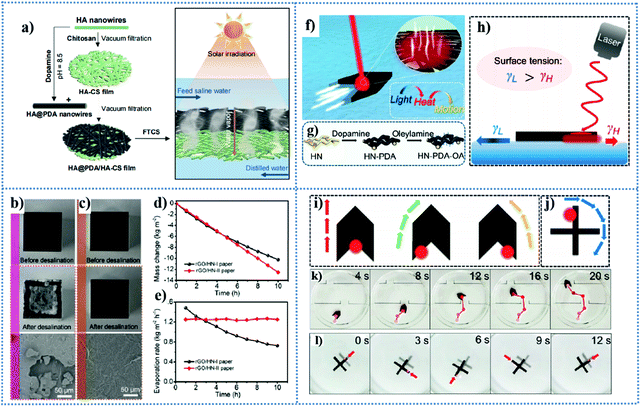 | ||
| Fig. 6 (a) Schematic illustration depicting the fabrication of HAP@PDA/HAP-CS bilayer photothermal film and PMD based on this bilayer structure and solar-driven desalination application of the retardant photothermal film. Reproduced from ref. 172 with permission from The Royal Society of Chemistry, copyright [2020]. (b–e) Solar desalination performance of RGO/HAP paper. (b) Digital images and SEM micrograph of the hydrophilic RGO/HAP-I paper before and after desalination. (c) Digital images and SEM micrograph of the hydrophobic RGO/HAP-II photothermal paper before and after desalination. (d) Mass change and (e) evaporation rate of an aqueous solution containing 3.5 wt% NaCl under 1 sun illumination using the hydrophilic RGO/HAP-I photothermal paper (black curve) and hydrophobic RGO/HAP-II photothermal paper (red curve). Reproduced from ref. 173 with permission from the American Chemical Society, copyright [2020]. (f) Schematic illustration of the HAP–PDA–OA paper-based actuator. (g) Preparation process of the HAP–PDA–OA paper. (h) Schematic illustration of the controllable motion triggered by the Marangoni flow. (i and j) Arrow-shaped and cross-shaped actuator made from HAP–PDA–OA paper and its different moving directions controlled by irradiating NIR light at specific locations. (k and l) Arrow-shaped and cross-shaped actuator move along a specific and preplanned path under the control of the irradiating NIR light. Reproduced from ref. 174 with permission from the American Chemical Society, copyright [2019]. | ||
In the process of pursuing high energy/evaporation efficiency from the perspective of thermal management and water transport control, one of the main challenges that must be considered is the accumulation and crystallization of salt on the surface of the evaporator, which will impair its water evaporation performance and long-term stability.167,175,176 Thus, to solve this problem, Qin et al.176 fabricated a salt-resistant hydrophilic/hydrophobic Janus evaporator. To avoid salt deposition, the sodium oleate-modified hydrophobic ultralong HAPNW layer can prevent saline water permeation, and the air-laid paper can provide fast water-transporting channels to facilitate salt exchange. In their work, black nickel oxide (NiO) nanoparticles with high light-absorbing and energy converting ability were deposited on HAP-based paper. Although the water transportation ability of the Janus HAPNW/NiO photothermal paper was slightly weaker than that of the hydrophilic HAPNW/NiO photothermal paper, it still exhibited a high water evaporation efficiency of 83.5% under 1 kW m−2 irradiation, while obtaining long-time stable water evaporation and salt-rejecting performance in the process of desalination using simulated seawater (3.5 wt% NaCl) without obvious salt accumulation on its surface. Similarly, inspired by the idea of hydrophobization treatment, Xiong and co-workers173 utilized reduced graphene oxide (RGO) and ultralong HAPNWs to construct salt-rejecting photothermal paper for solar energy-driven water evaporation. One of the highlights of this study is that the hydrophilicity/hydrophobicity of the RGO/HAP photothermal paper can be adjusted via the degree of reduction of graphene oxide during thermal treatment. The hydrophilic RGO/HAP photothermal paper (RGO/HAP-I) and hydrophobic RGO/HAP (RGO/HAP-II) were obtained by heat treatment under vacuum at 150 °C for 2 and 6 h, respectively. As shown in Fig. 6d and e, although the water evaporation rates of the RGO/HAP-II paper were lower than that of RGO/HAP-I initially, RGO/HAP-II paper exhibited a better linear relationship between mass change and time in the desalination process and maintained a stable evaporation rate throughout the entire desalination process. Therefore, no significant salt deposition occurred on the surface of the hydrophobic RGO/HAP-II paper after continuous solar desalination for 10 h (Fig. 6c), whereas the opposite was observed for the hydrophilic RGO/HAP-I paper (Fig. 6b), which demonstrated that the hydrophobic RGO/HAP-II paper has an outstanding salt-rejecting performance and can be used for long-term desalination.
Considering the drawbacks of a hydrophobic surface for reducing salt accumulation such as sacrificing the water evaporation rate, more efficient methods have been employed to solve the problem of salt deposition, for example, photothermal membrane endowing superhydrophobicity,167 contactless steam generation177 and others.175 In summary, the HAP-based paper is promising for constructing solar-driven interfacial water evaporation systems, although there are not enough relevant studies. However, as can be seen, the existing challenges require more efforts to solve for promoting the prosperity of this industry.
To further extend the applications of HAP-based photothermal paper, Yang and co-workers explored its application for controllable light-driven self-propelled motion.174,178 In their initial design, the movements of self-propelled actuators were driven by spontaneous liquid flow utilizing the Marangoni effect. According to the previous research results, light-to-heat conversion based on HAPNWs can be used to generate localized heating and spatial gradient of surface tension for the formation of Marangoni propulsion (Fig. 6h). Another crucial reason is that photothermal paper based on ultralong HAPNWs has good mechanical properties, high flexibility and excellent processability, which provide possible opportunities to design the shape and structure of photothermal paper for regulating the movement behaviors of actuators (Fig. 6i–l).174 PDA was employed as a modified coating of HAPNWs to obtain photothermal paper and oleylamine (OA) was further introduced to achieve superhydrophobic HPA–PDA–OA paper (Fig. 6h). The superhydrophobic structure reduced the fluid drag when the floating paper moved on the surface of water via the formation of a thin air layer between the floating superhydrophobic paper and water surface. It was proven that this HPA–PDA–OA paper has high light absorption efficiency and rapid photothermal response, and its movement behaviors can be manipulated by controlling the irradiated light. In addition, this group designed another light-operated dual-mode propulsion, which can transform between light-driven motion and vapor-enabled propulsion modes by increasing the light power density, adapting to the failure of the Marangoni effect in water containing a surfactant.178
An ultralong HAPNW-based layered inorganic paper with high flexibility, superhydrophobicity and fire resistance was successfully applied for oil absorption.165 It is worth mentioning that the nacre-like layered structure of the inorganic paper self-assembled by HAPNWs can increase the roughness and enhance the resistance to mechanical destruction. Wen et al.179 reported a layered superhydrophobic inorganic paper made from HAPNWs loaded with ZnO particles, followed by modification with 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDS). The resulting inorganic paper (PFDS-paper@ZnO) could rapidly absorb oil colored red with a dye from the mixture of oil and water (Fig. 7a). As the red paper returned to its original color after burning (Fig. 7b), the superhydrophobicity of the paper disappeared due to the decomposition of PFDS. However, the HAP nanowires and ZnO particles were well preserved, which could be modified with PFDS to recover the superhydrophobicity (Fig. 7c). A water droplet retained its spherical shape on the surface of the cycled paper and no apparent difference was observed in the structure of the paper after 35 cycles179 (Fig. 7d). Moreover, the burnt paper exhibited superhydrophilicity in air and underwater superoleophobic properties, which can be used for the efficient separation of oil-in-water emulsions. In another work proposed by Wen and co-workers, they fabricated a multifunctional superhydrophobic inorganic paper composed of carboxymethyl cellulose (CMC), HAP and ZnO without extra chemical modification.180 The oil adsorption and wettability of the paper could be maintained even after 30 cycles. Yang and co-workers181 fabricated a fire-resistant and superhydrophobic paper by utilizing ultralong HAPNWs as building blocks for decorating magnetic Fe3O4 nanoparticles and coating a polydimethylsiloxane layer (Fig. 7e). The resultant paper was used for the selective separation of oil/water with high performances, which was fabricated into a vessel-type shape for magnetically controlled oil collection (Fig. 7f). The HAP@Fe3O4@PDMS inorganic paper had a high separation efficiency of 99.6% and a good recycling performance for 10 cycles with higher than 99.0% efficiency, and simultaneously showed a high permeation flux of 2835.9 L m−2 h−1 due to its porous structure constructed by ultralong HAP nanowires.
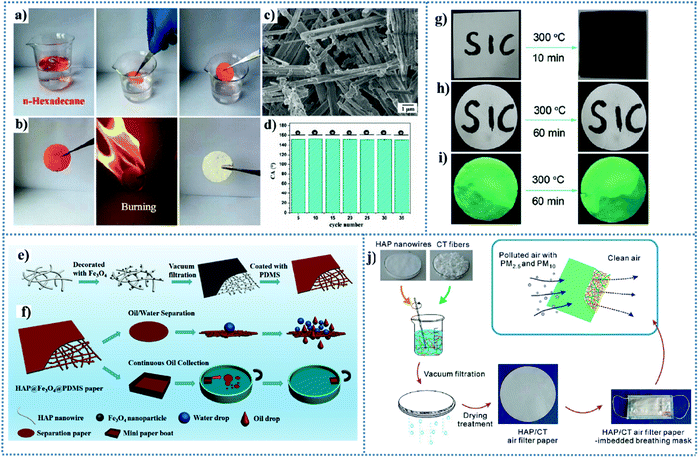 | ||
| Fig. 7 (a–d) Oil adsorption–combustion test. (a) Photographs showing the oil adsorption process of n-hexadecane by using PFDS-paper@ZnO taken at intervals of 5 s (n-hexadecane was dyed by Sudan red IV for clear observation). (b) Photographs showing the combustion process of hexadecane-containing superhydrophobic paper. (c) SEM images of PFDS-paper@ZnO after oil adsorption–combustion test. (d) Change in CA of water droplet during repairable test. One experiment consisted of 5 complete cycles when the oil-containing paper was burnt and immersed in PFDS ethanol solution in the repair experiment. Reproduced from ref. 179 with permission from The Royal Society of Chemistry, copyright [2018]. (e) Schematic illustration of the fabrication process of HAP@Fe3O4@PDMS paper and (f) its application as filter paper for oil/water separation and magnetically driven oil collection. Reproduced from ref. 181 with permission from the American Chemical Society, copyright [2017]. Digital images of two types of paper sheets before and after thermal treatment: (g) common commercial paper made of plant cellulose fibers, (h) HAPNW:Tb3+ paper, and (i) HAPNW:Tb3+ paper under UV irradiation (∼365 nm). Reproduced from ref. 188 with permission from the American Chemical Society, copyright [2019]. (j) Schematic illustration of the ultralong HAP nanowires and CT fibers as building blocks for the fabrication of the HAP/CT air filter paper through a simple vacuum filtration process, and subsequently the as-prepared HAP/CT air filter paper-imbedded breathing mask is used for highly effective removal of PM2.5 and PM10. Reproduced from ref. 189 with permission from The Royal Society of Chemistry, copyright [2019]. | ||
The HAPNW-based paper has been reported as a substrate for supporting some special catalysts for the catalytic degradation of organic dyes or toxic chemicals in recent years. For example, gold nanoparticles (AuNPs) with excellent catalytic activity were loaded on HAPNW to form HAPNW/AuNP-layered catalytic paper.182 The composite paper was proven to catalyze the continuous flow of 4-nitrophenol degradation with high catalytic efficiency (100%) and exhibited a high degradation efficiency for organic dyes (rhodamine B (RhB) and methyl orange (MO)). In a previous report, fabricated Ag3PO4/HAP composites exhibited photocatalytic activity to decompose rhodamine B (RhB), methyl orange (MO) and methylene blue (MB) for water treatment.183 In fact, HAPNW-based paper with a porous layered network structure not only provides abundant sites for anchoring functional nanoparticles but is also beneficial to control and adjust the water transportation rate and permeability.184 Moreover, synthesized HAP adsorption membranes can efficiently remove organic micropollutants,185 indicating the good development prospect of HAP materials in water treatment. In addition, Ca2+ and OH− in hydroxyapatite have ionic exchange ability with some heavy metal ions (such as Pb2+) and anions (such as F−), and PO43− shows reasonable affinity towards certain ions,186,187 which give hydroxyapatite-based materials great potential opportunities for water purification.
Given that HAPNWs possess a large surface area and abundant Ca2+ ions, PO43+ ions, and hydroxyl groups on their surface, HAP-based paper with a 3D porous structure has the ability to capture particles and load functional agents.189 Therefore, HAP paper can be used not only to successfully manufacture antibacterial HAP paper by decorating regents with antibacterial activity but also to manufacture HAP-based air filtration products. Xiong and co-workers189 reported a new type of air filter paper with a porous and loose network structure by employing ultralong HAPNWs and cotton (CT) fibers as raw materials for the efficient removal of PM2.5 and PM10. The fabrication processes of the HAP/CT air filter paper and the homemade breathing mask made from the HAP/CT air filter paper are shown in Fig. 7j. Benefiting from the as-prepared HAP/CT paper with high porosity and mesoporous structure, the HAP/CT air filter could allow air to easily pass and had a high affinity with particles. Therefore, the removal rate of PM2.5 and PM10 was more than 95% after optimizing the ratio of HAP and CT. Besides the inherent advantages of HAP-based paper for air filtration, another group utilized the synergistic effect of HAPNWs and NH2-UiO-66 to achieve high removal efficiencies for PM2.5 and PM10.191 NH2-UiO-66, a type of MOF, exhibited excellent removal ability for harmful particles and dyes, which was attributed to its high surface area, porous structure and functional groups. More interestingly, the resultant HAP@NH2-UiO-66 exhibited stable superhydrophilicity in air and underwater superoleophobicity (θoil > 150) without any further chemical modification, which indicates that it can be applied for the separation of oil-in-water emulsions.191
Cellulose paper can be doped with lanthanide ions for anti-counterfeiting, and HAPNW-based inorganic paper with luminescence has been produced using lanthanide-ion-doped ultralong HAPNWs (HAPNWs:Ln3+).188 Compared to cellulose paper as a raw material with flammability, after heat treatment at 300 °C for up to 60 min, the luminescent HAPNW:Tb3+ inorganic paper did not change significantly as a writing paper, and its photoluminescence color and intensity generally remained the same as the pristine properties, indicating high thermal stability and excellent fire resistance of the inorganic paper (Fig. 7g–i). Under irradiation with a UV lamp (∼365 nm), the HAPNW:5% Tb3+ paper and HAPNW:5% Eu3+ paper exhibited strong green and red colors, respectively. Therefore, the weight ratios of HAPNW:Eu3+ and HAPNW:Tb3+ can be varied to achieve tunable photoluminescence properties under a UV lamp (∼365 nm). In addition, according to the experience from the previous exploration, HAPNWs as a raw material provide a good space for chemical modification, and in this case, it is naturally available to endow photoluminescent inorganic paper with water-proof properties.
3.2 Carbon-based paper and its multifunctional applications
In the carbon family, graphene exhibits many unique properties, including excellent mechanical performances, with a breaking strength of about 125 GPa, and extraordinarily high thermal conductivity (5300 W m−1 K−1), high electrical conductivity (104–106 S cm−1) and high theoretical surface area of 2630 m2 g−1.192–195 Based on these advantages, graphene paper and films with flexibility and satisfactory mechanical properties have been fabricated, and have attracted considerable interest in multiple fields, including flexible energy storage devices such as supercapacitors and various types of batteries,16,196,197 thermal management, and EMI shielding.198 For example, for the first time, Wallace et al. studied the lithium-ion battery (LIB) performances of graphene paper.196 In 2019, Karthick et al.16 summarized the strategies for the synthesis of graphene paper and its properties as flexible devices in both energy storage and conversion system. Xu et al. reported the preparation of multifunctional graphene paper with a compact density of 1.85 g cm−3via the direct densification of RGO foam, possessing high thermal conductivity, high electrical conductivity and excellent EMI shielding effectiveness.192 The physical and chemical properties of paper are largely related to its synthetic method. At present, the methods for the synthesis of graphene or GO paper can be roughly divided into vacuum-assisted filtration,9,10,199–201 direct evaporation,202 roll to roll processing,203 mechanical pressing of graphene aerogel,197 and densification of RGO.192 To the best of our knowledge, GO is easily dispersed in an aqueous medium due to its surface-containing abundant oxygen-containing groups, which can facilitate paper-making methods; therefore, GO paper is the preferred precursor to prepare graphene paper. Fig. 8 displays several graphene papers fabricated by different methods and the corresponding SEM images of the resultant graphene paper. | ||
| Fig. 8 (a) Photograph of two pieces of free-standing graphene paper fabricated via vacuum filtration of chemically prepared graphene dispersions, followed by air drying and peeling off the membrane. Front and back surfaces shown. (b) Top-view SEM image of a graphene paper sample showing a smooth surface. (c and d) Side-view SEM images of a ca. 6 mm thick sample at increasing magnification. Reproduced from ref. 9 with permission from WILEY-VCH, copyright [2008]. (e) Schematic representation of a proposed self-assembly process of GO film by the evaporation of GO suspension, as well as the following graphitization process. Reproduced from ref. 202 with permission from WILEY-VCH, copyright [2014]. (f) Steps in the preparation of flexible, free-standing graphene paper starting from graphite sheets. Reproduced from ref. 201 with permission from Elsevier, copyright [2018]. (g) Schematic illustration of the pressing process of graphene paper. (h) SEM image of graphene aerogel (GA) with inset showing an optical photograph of GA. (i) SEM image of GA under 50% compression. (j) SEM image of a section of TAGP, and the three insets show the flexibility of TAGP. (k) Bent section of TAGP. (l) In-plane view of the folded graphene sheets. (m) Cross-sectional view of the folded graphene sheets. Reproduced from ref. 192 with permission from The Royal Society of Chemistry, copyright [2018]. | ||
In addition, GO paper can be converted from a pristine insulating state at room temperature to a conductive state after a reduction process, which is attributed to the removal of a large number of oxygen-containing groups on GO. Taking advantage of this behavior of recoverable conductivity, GO paper has been proven to have the potential for developing fire alarms. Furthermore, the emergence of numerous actuators based on graphene paper not only caters to the current development trend of intellectual technology but also further draws attention to the structural design of graphene paper and the mechanism of interlayer water permeability. In this section, we selectively present the four main applications of graphene paper to show that paper-like graphene as a novel type of paper is enriching the applications of flexible 2D materials in many fields.
One strategy that has been proven effective is to construct additional heat flow channels by embedding thermally conductive materials between graphene layers.205 It has been proven that three-dimensional (3D) interconnected thermally conductive networks attained by assembling two-dimensional (2D) graphene paper and fillers with high thermal conductivity can enhance the thermal conductivity of paper-based thermal conductive systems owing to the more effective transmissions of phonons.213 To the best of our knowledge, zero-dimensional (0D) nanoparticles such as nanodiamonds (ND)214 and alumina NPs209 have been employed as thermally conductive fillers to insert into horizontal graphene layers. Nan and co-workers constructed a 3D hybrid thermal management nanocomposite paper through the self-assembly of cationic poly(diallyldimethylammonium chloride) (PDDA)-functionalized GO and negatively charged ND particles via electrostatic interactions.214 The ND particles can effectively fill the spaces and edges between the GO nanosheets and connect adjacent GO sheets, resulting in the formation of a 3D interconnected graphene-nanodiamond architecture. The results indicated that the optimized thermal conductive paper exhibited satisfactory in-plane thermal conductivity of 16.653 W m−1 K−1 and showed a significant improvement compared with that of pure GO paper of 3.108 W m−1 K−1 and PDDA-functionalized GO paper of 5.467 W m−1 K−1. Similarly, Hong et al.209 employed a spin-assisted layer-by-layer deposition method to assemble GO nanosheets and functionalized alumina nanoparticles, followed by thermal reduction treatment for the construction of RGO/alumina stacked films. The process of thermal treatment can efficiently remove some functional groups and polymeric residues around the GO nanosheets for restoring the in-plane thermal conductivity, which is ascribed to formation of more sp2 hybridized carbon structures, providing more effective channels for phonon transport.209,215,216 Moreover, the resultant film presented a ladder-like architecture (Fig. 9a), which can facilitate the thermal transport, especially in cross-plane direction, leading to a remarkably high in-plane thermal conductivity of 565 W m−1 K−1 and a cross-plane thermal conductivity of 18.1 W m−1 K−1. In contrast, the cross-plane thermal conductivity of the RGO-only film was as low as 0.12 W m−1 K−1.
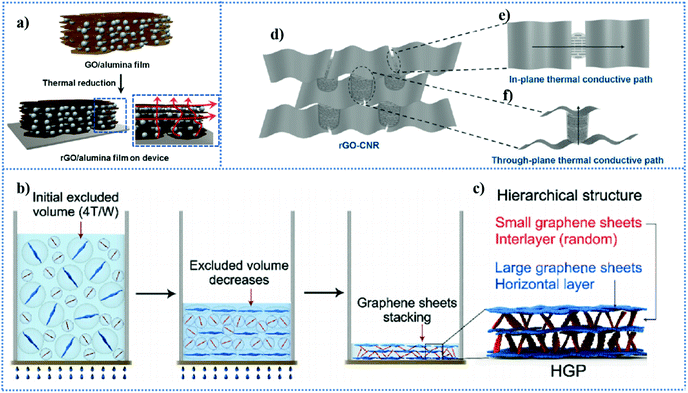 | ||
| Fig. 9 (a) Schematic illustration of fabricated RGO/alumina-complexed films and the subsequent thermal reduction process. Reproduced from ref. 209 with permission from The Royal Society of Chemistry, copyright [2019]. (b) Schematic illustrating the assembly behavior of a mixed dispersion containing two different sizes of graphene sheets during a rapid filtration process, leading to the formation of (c) hierarchically structured graphene paper. Reproduced from ref. 205 with permission from Elsevier, copyright [2021]. (d–f) Schematic illustration of graphene-based composite films with 3D hierarchical thermal conductive network: (d) integral RGO–CNR composite film; (e) scheme of the thermal conductive network in the in-plane direction; and (f) scheme of the thermal conductive network in through-plane direction. Reproduced from ref. 217 with permission from the American Chemical Society, copyright [2018]. | ||
In addition to the nanoparticles mentioned above, assembling graphene and some 2D materials (such as MXenes,216 boron nitride nanosheets (BNNSs)207,218 and certain size graphene sheets205) is an efficient pathway to fabricate carbon-based composite paper with high thermal conductivity. BNNS has the advantages of good electronic insulation, high thermal conductivity and strong stiffness, and thus is regarded as a candidate for the construction of thermal management materials with electrical insulation. However, pure BNNS paper generally shows low thermal conductivity due to the weak interaction between adjacent BNNSs.218 Interestingly, it was found that BNNS and GO possess a similar frequency range for phonon dispersion.218 Inspired by the interface and orientation of natural nacre, Yao and collaborators218 filled flexible GO sheets into the gaps between adjacent BNNSs, and a strong interaction was formed between them, resulting in reduced thermal boundary resistance owing to phonon spectral match. Therefore, the resulting BNNS–GO paper exhibited a much higher in-plane thermal conductivity of 29.8 W m−1 K−1 than that of pure BNNS paper and GO paper. In addition, another group integrated GO and boron nitride microplatelets (BNMPs) instead of exfoliated BNNSs to fabricate BNMPs/GO paper with high in-plane thermal conductivity of 10.3 W m−1 K−1 at 50 wt% BNMPs.207 Subsequently, Gao et al.205 prepared an all-graphene paper with high cross-plane thermal conductivity of 12.6 W m−1 K−1 after a common graphitization post-treatment, having promising application as thermal interface materials (TIMs). In fact, sp2-hybridized carbon materials with high thermal conductivity, including graphene, CNTs and carbon fibers, can be considered as good insert materials for constructing thermal management systems.205 Essentially, the crucial point to improve the cross-plane thermal conductivity of graphene paper is to construct an effective heat pathway between horizontal graphene layers by properly designing the arrangement of the inserted materials and graphene sheets. In the work reported by Gao et al.,205 the structure of the resultant graphene paper can be described as randomly arranged small graphene sheets intercalated into the loosely stacked horizontal layers formed by the stacking of large graphene sheets (Fig. 9b and c). Due to the small size of the graphene inserts, effective heat transfer pathways were successfully formed and the cross-plane thermal conductivity was significantly enhanced compared to pure GO paper.
To improve both the in-plane and through-plane thermal conductivity, connecting graphene sheets is an efficient approach for reducing the thermal resistance caused by the boundaries and edges between sheets. Based on this strategy, Meng et al.217 employed carbonized cellulose nanocrystals (CNCs) as a bridge to connect the boundaries and gaps between graphene in-plane and through-plane layers. CNCs and carbonized nanorods (CNRs) are both rod-like structures. Benefiting from their advantageous structure (Fig. 9d–f), the all-carbon paper exhibited high thermal conductivity not only in-plane (1820.4 W m−1 K−1) but also through-plane (4.596 W m−1 K−1). After high annealing treatment, CNRs connected two adjacent RGO sheets by covalent bonds. Recently, some groups utilized covalent bonding to improve the interface interaction between fillers and GO, which is meaningful for enhancing the thermal conductivity of composite materials.206,219,220 For example, Qu et al.206 prepared a flexible film with ultrahigh thermal conductivity and significant flame retardancy through covalent binding between the amino groups of amino-functionalized BP (BP–NH2) and carboxyl groups of GO, followed by reduction reaction. The covalent connection formed between adjacent GO nanosheets by the BP–NH2 sheets can eliminate the gap between adjacent graphene nanosheets, thereby forming a smooth thermal path to reduce the interface thermal resistance. Moreover, adjacent BP–NH2 will also form hydrogen bonds through amino groups, which can extend the heat conduction path and increase the thermal conductivity as much as possible. Consequently, the reduced BP–NH2/GO paper showed an ultrahigh in-plane thermal conductivity of 1085.74 ± 37.08 W m−1 K−1 and an ultrahigh thermal diffusivity of 1496.87 ± 51.11 mm2 s−1 when the amount of BP–NH2 was increased to 20 wt%. The results demonstrated that the paper assembled with unfunctionalized BP (20 wt%) and GO had lower in-plane thermal conductivity than the pure GO paper, which is ascribed to the severe agglomeration of the BP nanosheets in the GO film.206 Furthermore, this group also proved that same strategy can be successfully applied to assemble CNTs and BP–NH2 and achieved satisfactory results in another study.220
In 2008, Ruoff and co-workers firstly fabricated graphene-based supercapacitors using hydrazine-monohydrate-reduced GO as an electrode material.194 Owing to the fact that the graphene surface was not fully exposed to the electrolyte due to the aggregation of the individual graphene sheet during the reduction process, it merely showed a specific surface capacitance of 100 F g−1 in the KOH aqueous electrolyte. In fact, the restacking problem caused by the strong π–π interaction between graphene layers significantly decreased the accessible surface area for electrolyte ions,224,225 which has been one of the main challenges in the development of paper-shaped graphene SCs. Another challenge has revealed that a large number of defects generated in the fabrication process of graphene is beneficial for ion transmission but reduces the conductivity.193 Especially, when RGO as a precursor is assembled into 2D paper, followed by a reduction process to obtain graphene paper, the reduction method used will have a certain impact on the performance of the supercapacitor. This aspect will be described later. As summarized by Zhang and co-workers, the three main limitations of graphene electrodes used in supercapacitors are their low surface area, low conductivity and low capacitance.193
To solve the problem of low accessible surface area due to the restacking between graphene sheets, inserting “spacers” into the gap between graphene interlayers has been widely reported to be effective. At present, the materials used as spacers include CNTs,226–229 conducting polymers,230 water,231 and molecule spacers.225,232 In the work by Jiang and co-workers, CNTs were employed as interlayer hybrids and introduced into graphene nanomesh layers with defects, followed by hydrazine reduction, forming a CNT–graphene sandwiched structure with bidirectional ion diffusion ability in both the cross-plane and in-plane directions226 (Fig. 10a). In this case, in addition to acting as a spacer to prevent the stacking of graphene sheets, CNTs also increased the conductivity of the hybrid film. Based on its favorable structure, the resultant film exhibited excellent electrochemical performances, including a specific capacitance of 294 F g−1 at 5 mV s−1, excellent cycling performance (93% capacitance retention after 5000 cycles), high energy density of 26 W h L−1 and high volumetric capacitance of 331 F cm−3.226 Different from the horizontal-placed CNTs, Abdollahi et al.227 utilized a plasma method to controllably grow vertically aligned carbon nanotubes (VACNT) on the surface of activated reduced graphene oxide (a-RGO), forming a flexible hybrid VACNT/a-RGO paper with outstanding electrochemical performances for application as an anode in supercapacitors and lithium-ion batteries. Recently, Wu et al. intercalated functionalized CNTs into graphene layers by using molecules as glue to connect the RGO “bricks” and CNT “mortar” through covalent bonds.228 In brief, aniline polymerizes on the surface of CNTs to form a core–shell structured composite (CNT@PANI). The amino group on the surface of CNT@PANI, the phenolic hydroxyl group in TA, and the oxygen-containing functional group in GO can form a large number of hydrogen bonds and π–π accumulation, obtaining a three-dimensional multilayer structure. After reduction treatment, strong covalent bonds are formed among the three components (GO, TA, and CNT@PANI), and thus the obtained CNT@PANI/RGO/TA film exhibited excellent flexibility and toughness, and could be bent and folded into different shapes.228 The composite film showed excellent electrochemical performances, especially at low-temperature conditions. Even at −40 °C, it exhibited a high specific capacitance of 454.9 F cm−3 in a supercapacitor, which is equivalent to retaining about 83% of the capacitance delivered at room temperature.228
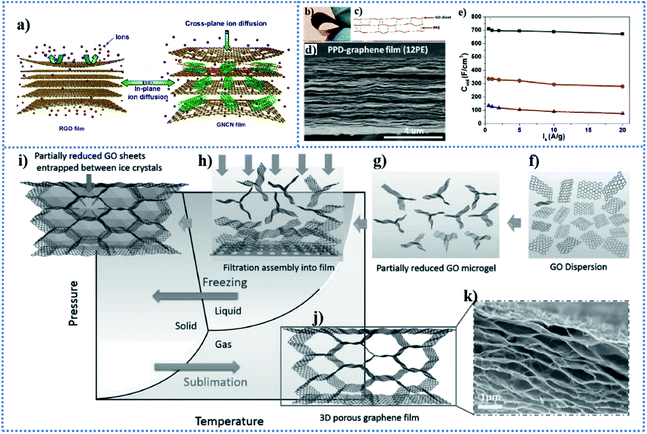 | ||
| Fig. 10 (a) Schematic illustration of the ion diffusion behavior of RGO film and porous GNCN film. Reproduced from ref. 226 with permission from Elsevier, copyright [2021]. (b) Optical image of flexible PPD–graphene film. (c) Schematic diagram of the micro model assembled by GO sheet and PPD. (d) High-magnification SEM images of PPD–graphene film. (e) Specific volumetric capacitance of sample measured from charge/discharge (CD). Black curve: PPD–graphene film (12 PE); red curve: PPD–graphene film (E); and blue curve: pristine graphene film. Reproduced from ref. 232 with permission from the American Chemical Society, copyright [2017]. Mechanism of forming a 3D porous microstructure. (f–j) Schematic illustration of the formation of a porous graphene film through prereduction, filtration assembly and freeze-casting. The water phase diagram shows the status of the aqueous solution during the different procedures. (k) Typical cross-section SEM image of a porous graphene film. Reproduced from ref. 221 with permission from WILEY-VCH, copyright [2016]. | ||
To obtain a high specific volumetric capacitance (Cvol), the gravimetric capacitance (Cwt) and density (ρ) need to be promoted as much as possible.232 However, there is a contradictory relationship in the process of increasing Cvol. On the one hand, the designed porous structure is used to provide more void space for electrolyte storage such as graphene foam, which helps to increase Cwt but is limits the density.232 On the other hand, a dense assembly of multilayer graphene can easily lead to a significant decrease in the surface accessible to ions and an obvious increase in the diffusion resistance of the electrolyte, leading to a limited Cvol. Focusing on this problem, Lian and co-workers intercalated p-phenylenediamine (PPD) molecules as nanoscale spacers into GO sheets, and adjacent GOs were connected by the amino group on the para position of p-phenylenediamine232 (Fig. 10c). After the PPD-reduced graphene film was compressed by capillary action and controlled mechanical force to remove the trapped volatile solvent, they obtained a film with a high density (1.55 g cm−3). No stacking effect occurred due to the presence of abundant PPD molecules, the results showed that a uniform extended spacing (∼1.1 nm) was formed between the graphene layers, which is greatly conducive to the diffusion of ions parallel to the graphene plane.232 The resulting interconnected channels facilitated the transport of ions throughout the PPD-reduced graphene film. In addition, the modification of the graphene layers by PPD also enhanced the surface wettability of the film. Balancing the two crucial factors of density and ion-accessible area, the resulting film exhibited an ultrahigh volumetric capacitance (711 F cm−3 at a current density of 0.5 A g−1, as shown in Fig. 10e), high power and energy densities and excellent cycling stability in aqueous electrolytes. The optical image and high-magnification SEM images of the PPD–graphene film are shown in Fig. 10b and d, respectively.
Another strategy to inhibit the aggregation and restacking of graphene nanosheets is assembling them into a 3D porous structure, which exhibits merits such as large specific area and sufficient porosity, contributing to fast mass transport and high energy densities.221,223,233 In recent years, a variety of methods have emerged for the construction of three-dimensional porous network structures. For example, Shao et al.221 utilized filtration assembly and freeze-casting technology to fabricate 3D hierarchical porous graphene films by using partially reduced GO. In this work, the continuous 3D porous graphene films (Fig. 10k) were formed based on a specific percolation threshold, which was needed for pre-reduction and to control the reduction time for the GO sheets.221Fig. 10f–j illustrate the mechanism of forming a 3D porous microstructure. The flexible all-solid-state supercapacitors produced by using the 3D interconnected porous RGO films as the active material showed excellent electrochemical performances, strong mechanical strength, and ultrahigh power densities. Furthermore, to realize the controllable assembly of ordered layered structures between graphene nanosheets for the construction of a 3D network structure, it is a good choice to introduce polymers to connect the graphene sheets with chemical bonds. For example, Cao et al.234 used the method of molecular level coupling to fabricate high-strength graphene-based films for flexible supercapacitors with a high Cvol. It is easy to form a stable molecular-level coupling between GO sheets and polyvinyl alcohol (PVA) molecules through hydrogen bonds. On the surface of Zn foil, they employed graphene as the blocks and PVA molecules as the cement, which were coupled by hydrogen bonds formed between the oxygenic functional groups of GO sheets and the hydroxyl groups on the PVA molecules.234 During this process, the 3D network structure was constructed through layer-by-layer assembly, and the GO sheets were synchronously reduced. Compared with porous RGO films and compact RGO films, the resulting RGO@PVA composite films showed enhanced mechanical properties owing to their ordered layered structure aligned by RGO and PVA. PVA is a commonly used polymer in gel electrolytes. When H2SO4 is introduced, the PVA/H2SO4 electrolyte layer between the RGO sheets can form fast ion transport channels, resulting in excellent electrochemical performances. Recently, polyoxometalates (POM) nanoclusters were shown to exhibit outstanding catalytic activity, ionic conductivity, faradaic charge storage ability and electrochemical behavior and were used as a functional cross-linker to assemble RGO nanosheets into macroscopic 3D interconnected macroporous POM–graphene frameworks (POM–GFs).233 The results indicated that the obtained POM–GFs films exhibited excellent-supercapacitor performances, including high gravimetric capacitance of 205 F g−1 and volumetric capacitance of 334 F cm−3 at 1 mV s−1.233 In addition, researchers have also paid attention to the changes in the morphology and interlayer spacing of stacked graphene, which are caused by the change in interlayer force driven by the removal of surface functional groups during the reduction of graphene oxide.224
In addition, pseudocapacitance materials not only can be used as spacers to expand the interlayer distance between graphene sheets for providing more ion transmission paths but can also greatly increase the capacitance due to their Faraday energy storage mechanism.193 At present, the pseudocapacitance active materials that have been widely studied include transitional-metal oxides,235,236p-phenylenediamine,237 polypyrrole,238 dopamine239 and others.240 It is also worth mentioning that heteroatom doping can efficiently enhance capacitance, such as nitrogen (N),241,242 phosphorus (P),232,243 and co-dopants (N, P, and S).244
Thus far, two elements, namely nitrogen and phosphorus, have been proven to play a crucial role in flame resistance.248–250 Kim et al.250 doped phosphorus into the edges of graphene nanoplatelets using red phosphorus and graphite via simple ball-milling. The graphene phosphonic acid obtained after the violent oxidation of graphene phosphorus showed good dispersion in many polar solvents and flame retardants and thus was applied as a fire retardant coating. Dong and co-workers248 reported the preparation of a flame-retardant graphene paper using hexachlorocyclotriphosphazene (HCCP, N3P3Cl6)-decorated GO, utilizing the synergistic flame retardant effect of nitrogen and phosphorus atoms. The thermal stability of the retardant GO–HCCP paper was significantly improved compared with that of GO paper. In addition, considering that GO has high reactivity and poor thermal stability because it contains a large number of functional groups,248 this group tried to remove some of the oxygen-containing functional groups to obtain reduced GO–HCCP (RGO–HCCP) paper by thermal reduction. The as-prepared RGO–HCCP paper exhibited more advantages in flame-retardant behavior than the unreduced GO–HCCP paper. However, flame-retardant additives containing halogenated compounds such as HCCP mentioned above are harmful to the environment and human health.206,249,251 Therefore, seeking new fire-resistant agents and simultaneously enhancing the sensitivity of fire-warning response are both necessary. Chen et al.246 employed phytic acid (PTA) as a phosphorus source to construct functionalized GO paper via evaporation-induced self-assembly for ultrafast fire-alarm application. As shown in Fig. 11a, a fire sensor was constructed by connecting a lamp, a battery and GO–PTA paper with wire.246 The fire response of both the GO paper and the GO–PTA paper doped with 25 wt% PTA was 0.5 s. However, comparing before and after doping, the alarm duration time of the GO–PTA paper was greatly improved to 258.0 s from 41.0 s for pure GO paper when 25 wt% PTA was introduced in the GO paper. Recently, Qu et al.247 synthesized a nano-filler (BP–MoS2) by ball milling black phosphorene (BP) and molybdenum disulfide (MoS2) and introduced it into GO for achieving a GO film with outstanding flame resistance and ultrasensitive fire response. MoS2 and BP are both 2D nanomaterials, and their synergistic effect of fire resistance endowed GO with excellent flame retardancy and ultra-fast fire warning response (about 1 s). It is worth noting in this report, a large number of fire-resistant nano-sheets (>20 wt%) and different proportions of MoS2 and BP in the nano-sheets significantly affected the mechanical properties of the composites. Therefore, the negative effects resulting from a large number of fire-resistant additives need to be considered.247
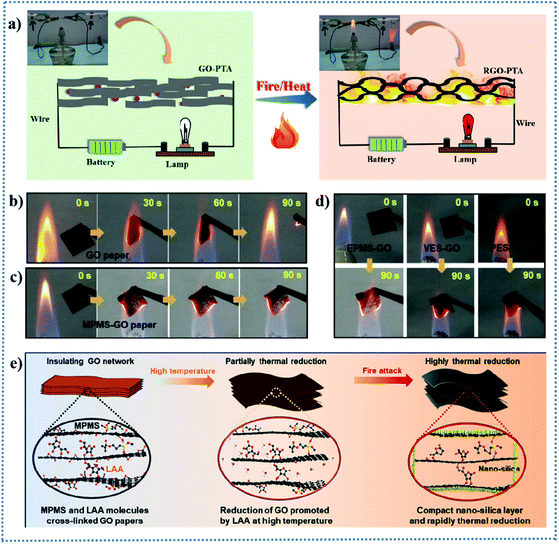 | ||
| Fig. 11 (a) Experimental circuit diagram and illustration of fire-alarm mechanism. Reproduced from ref. 246 with permission from Elsevier, copyright [2020]. (b–d) Flame-retardant properties and thermal stability of GO paper and various silane–GO papers. Combustion processes of (b) GO paper and (c) MPMS–GO paper, showing improved flame resistance after silane functionalization. (d) Photographs of various silane–GO papers before and after 90 s combustion test. Reproduced from ref. 249 with permission from Elsevier, copyright [2019]. (e) Schematic illustration of LAA and MPMS co-functionalization promoting the thermal reduction process of GO network. Reproduced from ref. 252 with permission from Elsevier, copyright [2020]. | ||
Another type of effective flame retardant additive is silane, which possesses alkoxy groups that can react with the hydroxyl groups of GO in an aqueous solution.249 For example, Huang and co-workers249 reported flame-retardant silane-grafted-GO (silane-GO) papers with enhanced flame resistance for a fast fire alarm response. Due to the bonding reaction between alkoxy and organic functional groups of the several types of silane molecules and hydroxyl groups of the GO sheets, cross-linking GO hydrogels with the 3D porous monolithic structure were formed after low-temperature hydrothermal treatment. Through assembly and arrangement, silane–GO paper with a compact and highly aligned structure was obtained. In the test of flame-retardant properties and thermal stability (Fig. 11b–d), the performances of the various silane–GO papers were significantly improved compared with that of the pure GO paper. The mechanisms can be explained based on the fact that a compact and solid nano-silica protective layer is uniformly formed on the surface of the GO sheet during the process of burning, efficiently protecting the GO paper from rapid thermal degradation. In addition, the silane-modified GO paper as a thermal sensor can rapidly detect flames (∼1.6 s) and maintain a continuous alarm response when a fire occurs. When connected to a thermal resistor, it could trigger an early warning response in a short time of ∼5 s. Moreover, this group devoted their efforts to further simultaneously enhancing the fire resistance and alarm response of the GO paper in their work.251 They produced flame-retardant GO paper using 3-mercaptopropyltrimethoxysilane (MPTS) as a modification agent. Benefiting from the MPTS molecules, which are silane molecules with sulfydryl terminal groups, they efficiently promoted the thermal reduction of GO compared with other silane molecules. The resulting MPTS-modified GO paper containing 10 wt% MPTS showed a fast flame detection response of only about 1.0 s, and the alarm response time was shortened to 232 s at 200 °C from 343 s at 200 °C for pure GO paper. Compared with their last work, the success of the above case is the chosen modification agent, which not only improved the thermal stability and flame resistance of GO paper but also promoted the thermal reduction of the GO network. Similarly, to pursue a low responsive temperature and further improve the early-warning fire response, Zhang and co-workers252 reported the preparation of a flame-retardant GO paper by employing 3-methacryloxypropyltrimethoxysilane (MPMS) as a modifier to improve the flame resistance and L-ascorbic acid (LAA) as a reducing agent to promote the thermal reduction of GO at high temperature. The LAA and MPMS co-functionalized GO network for promoting the thermal reduction process is shown in Fig. 11e. The optimized GO paper co-modified with MPMS and LAA not only could rapidly trigger a flame detection signal of ∼1 s but also release an early fire warning responses at a low temperature of ∼120 °C and reduce the alarm response time to ∼7 s at 300 °C. In contrast, the untreated GO paper triggered an alarm only when the temperature reached ∼211 °C, and generated an alarm response time of up to ∼100 s at 300 °C.
In addition to the phosphorus-containing and silicon-containing systems reported above, more agents will be explored and used to improve the thermal stability and thermal sensitivity of GO paper for extending its practical application in fire alarms. For example, according to the results reported by Yuan et al., a GO network modified with boric acid exhibited excellent results.245 In addition, Yu et al.253 introduced functionalized silica nanoparticles in a GO-based paper, achieving a simultaneous enhancement in mechanical strength, stiffness and flame resistance in GO paper.
Earlier, the bilayer paper reported by Park and co-workers, composed of GO and COOH-functionalized multi-walled CNTs by sequential filtrations, could curl inward or outward depending on the changes in relative humidity and/or temperature.17 In this work, the mechanism of the bilayer paper was not fully understood, but based on thermal gravimetric analysis, it was speculated that it was caused by the different amounts of water between the two layers under the relative humidity or temperature. Subsequently, taking advantage of the difference in hydrophilicity between GO and RGO, anisotropic GO/RGO bilayer paper was fabricated via the partial gradient photoreduction of GO using several methods. For example, Han et al.259 reported the fabrication of moisture-responsive GO/RGO bilayer paper by employing focused sunlight as the light source for self-controlled photoreduction (Fig. 12a–d). The moisture-responsive mechanism can be could be explained by the fact that GO and RGO exhibit different water molecule adsorption capacities under humid conditions.259 The obvious variation in the interlayer spacing of the GO sheets under different humidity provided strong proof for the mechanism explanation. Interestingly, smart actuators based on the bilayer paper were developed, including a claw, an orientable transporter, and a crawler paper robot.259 For example, two GO/RGO “smart claws” could respond to humidity and bend when approaching a sweaty human finger (Fig. 12e and f). Based on the property of anisotropic water-molecule adsorption for smart actuators, there are also some reports that further explored the gradient reduction of graphene oxide to obtain anisotropic Janus GO/RGO paper, such as laser interference260,261 or laser writing,262 reductions triggered by different reduction and oxidation properties,263 unilateral ultraviolet (UV) irradiation,264 chemical reduction with hydriodic acid (HI)265 and others.266 Among them, compared with laser writing and sunlight irradiation, Han and co-workers264 further found that UV is conducive to the large-scale, efficient, and simple production of bilayer GO/RGO paper with a more uniform structure, resulting in a stable response and performing complex actions after external stimuli.
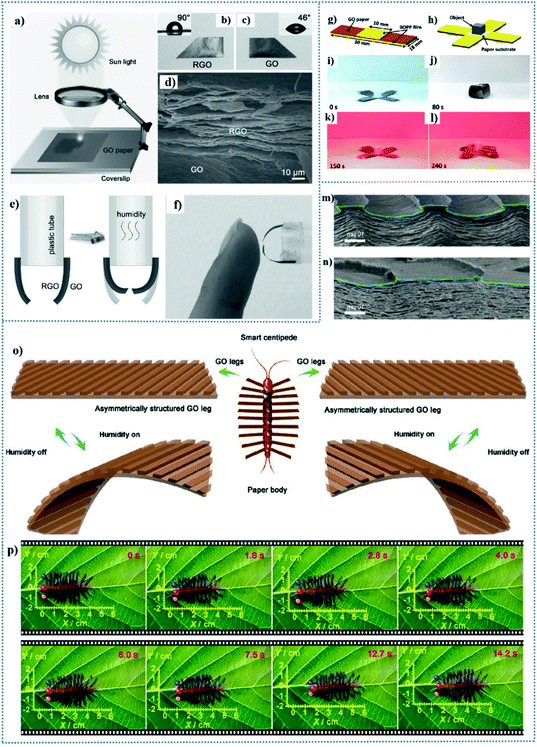 | ||
| Fig. 12 (a) Schematic illustration of the fabrication of GO/RGO bilayer papers using focused sunlight reduction. (b and c) Photographs of the resultant GO/RGO bilayer paper viewed from the front side (b) and reverse side (c). The insets showed water droplet contact angles of both sides. (d) SEM image of the section view of GO/RGO bilayer paper. (e and f) Moisture-responsive performance of a smart claw. (e) Schematic illustration of the structure of the smart claw. (f) The smart claw is very sensitive to moisture: it could bend when approaching a sweaty human finger. Reproduced from ref. 259 with permission from WILEY-VCH, copyright [2015]. (g) Schematic illustration of one foot of the intelligent robot. (h) Schematic illustration of the intelligent robot. (i) Optical photo showing the initial state of the intelligent robot. (j) Optical photo showing the protection mode of the intelligent robot responding to humidity. (k) Optical photo showing the intelligent robot responding to NIR light. (l) Optical photo showing the weightlifting mode of the intelligent robot responding to NIR light. Reproduced from ref. 268 with permission from the Royal Society of Chemistry, copyright [2017]. (m and n) Top and section view SEM images of structured GO film with periods of 40 μm (m), and 100 μm (n). Centipede-like soft mini-robot based on the smart GO paper. (o) Schematic illustration of moisture-responsive smart legs of the centipede mini-robot. (p) Snapshots of the crawling centipede mini-robot with smart GO legs. Reproduced from ref. 255 with permission from WILEY-VCH, copyright [2019]. | ||
Benefiting from its abundant oxygen-containing groups, GO paper or film enables rapid adsorption and ultrafast transmission of water molecules.264,267 Not limited to RGO with a moisture-inert property, GO can also be coupled with other moisture-inert material layers to fabricate moisture-responsive actuators that can deform due to strain mismatch at the bilayer interface.255 For example, Chen and co-workers reported a bilayer film based on GO paper and biaxially oriented polypropylene (BOPP) for a multi-response actuator.268 The humidity response stemmed from the disparity in affinity between GO paper and BOPP for water molecules, while the near NR light response was driven by the mismatched thermal expansion caused by the thermohydration effect of GO during heating. Based on the GO/BOPP film, the group designed an intelligent robot and a bioinspired helical actuator. As shown in Fig. 12g–l, the four arms of the intelligent robot gathered to protect objects as the humidity increased. Under the irradiation of infrared light, the four arms of the intelligent robot opened, and then showed a weight-lifting mode, which could lift objects. In addition, Wang et al.254 employed a CNT/PDMS layer with high photothermal properties and hydrophilic GO layer to produce a reversible soft actuator that is stimulated by light, thermal, and humidity. The resulting actuator displayed fast response performances and stable reversible bending deformation abilities. For example, the reversible bending motion of the bilayer membrane indicated that it only took 2.48 s to reach the maximum bending angle and it returned to its original shape within 9.65 s after the irradiation stopped. After 400 cycles, the double-layer film could reach the maximum bending angle (about 90°) in 2.5 s and quickly return to its original state after the photothermal stimulus was removed.
In brief, the key to the deformation of bilayer-structured actuators is to trigger stress mismatch by the asymmetric responsive behaviors on the two sides when they are subjected to external stimuli. In addition to the bilayer structure, there is another mode for the asymmetric response by pursuing the structure and morphology of smart paper. For example, Cheng and co-workers designed a single graphene oxide (GO) thin film for a multi-responsive actuator with an asymmetric surface structure on both sides via the evaporation induction of GO sheets.269 The actuator could reversibly deform in response to moisture, thermal, and infrared light stimuli, which is attributed to the asymmetric response caused by the different morphologies and roughness on both sides of the GO film. Furthermore, laser writing RGO on the smooth side of graphene can form a built-in integrated sensor for the real-time detection of its own deformation behaviors. Recently, another group constructed a fold nanostructured GO film as a moisture actuator via a simple soft template method on poly(dimethylsiloxane) (PDMS) gratings with variable periods instead of coupling other materials or building bilayer structures.255 In the scanning electron microscopy (SEM) images of the resultant GO film (Fig. 12m and n), the ordered grating structures could be clearly seen. The additional wrinkles caused by the grating structure can promote the absorption and transportation of water, which is considered to be the “quantum tunneling fluidics effect”, resulting in an asymmetrical moisture response on both sides of the GO film. Interestingly, the GO films with chiral twisting behaviors obtained by designing the orientations of the grating structure were employed as flexible legs to assemble a centipede mini-robot (Fig. 12o). Consequently, the simulated centipede could walk in a straight line at a uniform speed, which was driven by periodic twisting of the legs depending on the rhythmic switching of humidity (Fig. 12p). In addition, the designed smart leaf shape based on the structured GO film could catch a living ladybug and the fabricated large GO smart paper exhibited curling behavior on the palm.
4. Composite paper
In the previous section, we selectively introduced the typical applications of three types of paper made of three raw materials (cellulose fiber (CF), HAP, and carbon) in the energy and environment fields, depending on the characteristics of the paper. Among them, many researchers further explored the inherent advantages of the three raw materials and designed many hybrid papers with excellent performances or unique structures through the skillful hybrid assembly. In this section, we use some typical cases to demonstrate that hybrid paper, as an extension and expansion of the three basic papers, greatly enriches the construction and application of paper. At present, hybrid paper can be simply divided into CF/carbon, carbon/HAP, and HAP/CF.Composite paper combining cellulose and carbon derivatives has greatly attracted attention for application in flexible devices. As mentioned earlier, active carbon materials (CNTs, RGO, graphite, etc.) were introduced on the surface of cellulose paper, obtaining humidity and strain/pressure sensors. In another example, Zhu et al.270 designed a paper-based humidity sensor with a unique network structure formed via the self-assembly of conductive 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized cellulose fibers (TOCFs) and CNTs (Fig. 13a and b). Different from some strategies involving the hydrophilization of CNTs to improve the adsorption of water molecules, the functionalized cellulose fibers can provide more sites for the adsorption of water molecules and the composite network can amplify the humidity change signal via a more sensitive response for the swelling of the TOCFs after the absorption of water. Benefiting from these advantages, the sensor displayed excellent linearity (R2 = 0.995) in the humidity range of 11% to 95% RH and showed a good humidity sensitive response of 87.0% at 95% RH. Dichiara et al. prepared CNT–cellulose composite papers via a simple, low cost and “green” layer-by-layer nanoassembly process.271 The resultant CNT–cellulose paper exhibited a sensitive response to stress/strain and water because the embedded CNT network formed electrically conductive pathways.
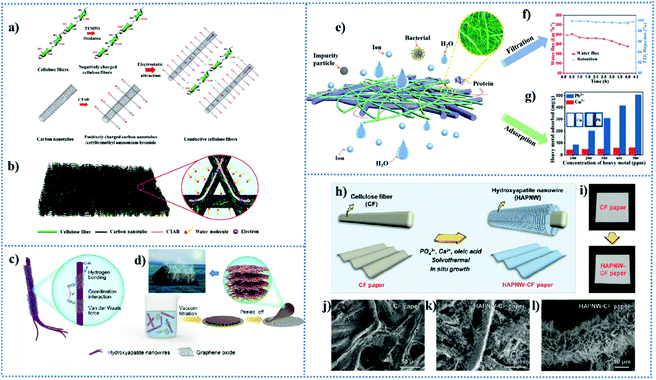 | ||
| Fig. 13 (a and b) Schematic illustrating the formation mechanism of the conductive cellulose fibers and the fiber network structure of the obtained paper sensor. (a) Schematic illustration of the preparation of conductive cellulose fibers by electrostatic attraction of negatively charged TOCFs and positively charged CNTs. (b) Schematic illustration of the network of the paper sensor based on the conformal conductive TOCFs/CNTs fibers. Reproduced from ref. 270 with permission from Elsevier, copyright [2021]. (c and d) Preparation process and characterization of GO/HN paper. (c) Hierarchical assembly of GO and HNs. (d) GO/HN paper with a layered structure prepared via the vacuum-assisted filtration process. Reproduced from ref. 173 with permission from the American Chemical Society, copyright [2020]. (e) Schematic illustration of the structure and filtration process of as-prepared HAPNW/CF filter paper. (f) Water flux and rejection rates of TiO2 nanoparticles at a concentration of 250 ppm during a period of 4 h using the HAPNW/CF filter paper with 60 wt% HAPNWs. (g) Adsorption amounts of Cu2+ or Pb2+ ions in CuCl2 or PbCl2 aqueous solutions with different initial concentrations. Reproduced from ref. 187 with permission from the American Chemical Society, copyright [2017]. (h) Schematic illustration of the in situ growth of HAPNWs on CF paper. (i) Photographs of the CF and HAPNW–CF papers with sizes of 2.5 cm × 2.5 cm. (j) SEM image of the CF paper. (k) SEM image of the HAPNW–CF paper. (l) SEM image of HAPNWs on an individual CF. Reproduced from ref. 275 with permission from the American Chemical Society, copyright [2021]. | ||
In fact, various carbon/cellulose paper flexible supercapacitors have been fabricated for energy storage. For example, Weng et al.94 introduced graphene nanosheets (GNS) on fiber paper through a simple filtration process, resulting in cellulose–graphene paper (GCP) with excellent flexibility. Due to the abundant functional groups on the surface of cellulose, GNS and CFs were attached by electrostatic interaction. GNS not only appeared on the surface of the paper but also filled the pores to form a conductive interwoven network. GCP as a flexible electrode exhibited good electrochemical properties, such as high capacitance per geometric area of 81 mF cm−2 and excellent cyclic stability after 5000 cycles. The role of CFs in the GCP-based supercapacitor electrode was to significantly absorb electrolytes and act as an electrolyte reservoir to facilitate ion transport.272 To fully utilize the CF network and the porous structure in the paper, Liu and co-workers coated GO nanosheets on the surface of CFs, followed by assembling GO sheets into microscale porous RGO networks in the pores of the paper via a hydrothermal process, leading to the formation of nanostructured RGO/CF composite paper.272 Benefiting from the nanostructure of the composite paper, it not only makes it easy for electrons to be transmitted in the entire CF network but also overcomes the problem of RGO aggregation in the pores of the paper and promotes the penetration of liquid ions. Therefore, RGO/CF paper with high flexibility and good mechanical properties has shown wide application in flexible electronics, energy-storage electrodes, sensors, etc.272 In another example, Yu et al.240 constructed RGO-coated cellulose fiber (RGO@CF) paper to adsorb organic dyes for the decolorization of solutions, where organic dyes were employed as pseudocapacitive materials to construct flexible supercapacitors based on dye-modified RGO@CF electrodes. In addition, CNTs as an excellent photothermal material were combined with cellulose paper to fabricate bilayer photothermal paper for water desalination and purification,8 which has been mentioned in the multifunctional applications of cellulose paper.
The hybrid paper assembled with HAP and carbon derivatives has the potential to be applied for solar energy-driven vapor generation and desalination. In one of the aforementioned reports, the HAPNWs were wrapped and cross-linked with GO via hydrogen bonding, coordination interaction, and van der Waals force during self-assembly, followed by a thermal treatment process, leading to RGO/HAPNW photothermal paper with a hierarchical porous structure (Fig. 13c).173 As shown in Fig. 13d, GO/HAP paper with a layered structure was prepared via the vacuum-assisted filtration process. The solar-driven evaporator combined the inherent advantages of the two components as well as their structural advantages, that is, HAPNWs have good heat insulation, RGO has excellent solar light absorption and photothermal conversion performances, and hierarchical porous structure provides channels for rapid water transport. Therefore, the two types of photothermal paper treated by different thermal processes possessed a high water evaporation rate and energy efficiency or outstanding salt-rejecting performance in the process of seawater desalination. Another typical combination of HAPNWs and carbon materials utilized HAP-based paper as a substrate and employed carbon materials as a photothermal layer deposited on the surface of the substrate. For example, Xiong and co-workers170 coated CNTs on HAP-based inorganic paper via simple vacuum-assisted filtration, leading to the formation of HAP/CNT photothermal paper for solar energy-driven photothermal seawater desalination and wastewater purification.
Cellulose fibers have shown great potential in water treatment due to their natural advantages such as low cost, porosity, excellent biocompatibility and biodegradability, high strength, and large modification space. HAP also acts as a material with high flexibility and biocompatibility, showing the promising application in water purification. According to recent reports, composite paper consisting of CFs and HAPNWs is already available and active in the field of water treatment. For example, Zhang et al.187 reported the preparation of HAPNW/CF filter paper containing polyamidoamine-epichlorohydrin (PAE) resin as a reagent for increasing the wet mechanical strength, showing a porous structure and superhydrophilicity. The pure water flux (PWF) of the complex paper was as high as 287.28 L m−2 h−1 bar−1 under cross-flow conditions, achieving an improvement of about 3200 times that of cellulose filter paper with the addition of PAE. To evaluate the water treatment capacity of the HAPNW/CF filter paper, the removal of TiO2 nanoparticles and bacteria in water respectively reached more than 98.61% and up to 100%, and the adsorption of methyl blue and Pb2+ ions gave satisfactory results with 273.97 mg g−1 and 508.16 mg g−1, respectively. The water purification performances of the composite paper are shown in Fig. 13f and g, including the water fluxes, rejection rates of TiO2 nanoparticles and adsorption amounts of Cu2+ and Pb2+ ions. In addition, the crucial physical properties such as porosity, PWF, and hydrophilicity can be changed with different HAPNW weight ratios. In further exploration, Zhang and co-workers273 tried to introduce another cellulose derivative, cellulose nanofibers (CNFs), as a barrier layer on the surface of a HAPNW/CF filter paper substrate for constructing a CNF–CF/HAPNW nanofiltration filter paper. The results demonstrated that the filter paper exhibited rejection rates above 95% for four different dyes and exhibited a relatively high rejection rate for Na2SO4 (75.7%) and NaCl (65.8%). Furthermore, to simultaneously improve both the water flux and separation efficiency for dye separation, double metal oxide (LDO) nanosheets with a high adsorption capacity for dye molecules were introduced via adsorption on the surface of HAPNWs and formed an LDO/HAP/CF porous network structure.274 The resulting nanocomposite filter paper achieved a high rejection percentage and water flux for Congo red (CR) with 98.3% and 736.8 L m2 h−1 bar−1, respectively.
Recently, Chen and co-workers275 reported a new type of hydroxyapatite nanowire–cellulose fiber (HAPNW–CF) paper via the in situ growth of ultralong HAP nanowires (HAPNWs) on CF paper (Fig. 13i). As shown in Fig. 13h, k and l, HAPNWs were radially aligned on the surface of CFs and formed a micro/nanoscale hierarchical structure. On the one hand, benefiting from the ability of ion exchange of HAP, the lanthanide ions (Eu3+ and Tb3+ ions) could be easily doped in HAPNWs by ion exchange with Ca2+ ions to obtain photoluminescent paper. On the other hand, the composite paper can be endowed with superhydrophobic properties by coating with PDMS for multifunctional applications such as oil/water separation. In addition, HAP/GO composite paper was designed as fire alarm wallpaper via the combination of the fire-resistance performance of HAPNWs and thermosensitive property of graphene oxide (GO), which are involved in the multifunctional applications of HAP-based paper.
5. Conclusion
In this review, from the common writable cellulose paper in daily life to the emerging inorganic paper and composite paper, we provided a new perspective on the development of two-dimensional paper-like materials. Firstly, cellulose paper is no longer limited to writing and packaging, but has become a base material with huge functional capabilities or is used as active material in certain applications, which is different from our traditional impression. Secondly, several inorganic raw materials for paper-making can be endowed with more application opportunities based on the advantages of 2D paper-like structures. Sometimes, the emergence of new two-dimensional material will bring new vitality to certain applications and even have a breakthrough impact. For example, HAP-based paper displays anti-naturally flame-retardant properties compared with traditional cellulose paper. This is based on its inherent advantages, and thus some researchers developed new applications based on HAP-based paper. Thirdly, with the booming development of 2D paper materials, the fabrication of paper with multifunctional applications has become a research hotspot. Among them, 2D paper-like composite materials skillfully composed with different inorganic raw materials possess unique advantages and will embrace more diversified development.Although considerable achievements have been made so far, more efforts are needed to solve the following three basic problems: (1) the fabrication methods and raw materials of paper need to be low cost, feasible, environmentally friendly, have mild synthetic conditions, and satisfactory properties. For example, graphene, a hotspot material in carbon materials, exhibits different performances caused by diverse synthetic methods. The synthetic methods for ultralong HAPNWs need to be low cost, environmentally friendly and suitable for mass production. (2) It is significant to explore more new applications or multifunctional applications of cellulose-based, inorganic-based and composite paper. (3) The combination modes of raw materials have an important impact on the performances of composite paper such as surface embedding, in situ growth, and incorporation of small constituent units at the molecular level. Herein, we did not cover all the emerging inorganic paper, composite paper and their corresponding applications in the environmental and energy fields. However, through the summary of the partial applications of three types of paper (cellulose, HAP, and carbon-based paper) and the introduction of composite paper, it is expected that this review article will arouse much interest in 2D paper-like materials and their multifunctional applications.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work is supported by the National Nature Science Foundation of China (No. 51735013).Notes and references
- Z. Li, X. Yang, W. Li and H. Liu, Carbohydr. Polym., 2019, 210, 350–363 CrossRef CAS PubMed.
- S. Ge, L. Zhang, Y. Zhang, F. Lan, M. Yan and J. Yu, Nanoscale, 2017, 9, 4366–4382 RSC.
- F. Guder, A. Ainla, J. Redston, B. Mosadegh, A. Glavan, T. J. Martin and G. M. Whitesides, Angew. Chem., Int. Ed., 2016, 55, 5727–5732 CrossRef PubMed.
- Z. Duan, Y. Jiang, M. Yan, S. Wang, Z. Yuan, Q. Zhao, P. Sun, G. Xie, X. Du and H. Tai, ACS Appl. Mater. Interfaces, 2019, 11, 21840–21849 CrossRef CAS PubMed.
- K. Xia, X. Chen, X. Shen, S. Li, Z. Yin, M. Zhang, X. Liang and Y. Zhang, ACS Appl. Electron. Mater., 2019, 1, 2415–2421 CrossRef CAS.
- S. Chen, Y. Song, D. Ding, Z. Ling and F. Xu, Adv. Funct. Mater., 2018, 28(42), 1802547 CrossRef.
- S. Chen, Y. Song and F. Xu, ACS Appl. Mater. Interfaces, 2018, 10, 34646–34654 CrossRef CAS PubMed.
- H. Huang, L. Zhao, Q. Yu, P. Lin, J. Xu, X. Yin, S. Chen, H. Wang and L. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 11204–11213 CrossRef CAS PubMed.
- H. Chen, M. B. Müller, K. J. Gilmore, G. G. Wallace and D. Li, Adv. Mater., 2008, 20, 3557–3561 CrossRef CAS.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- B. Q. Lu, Y. J. Zhu and F. Chen, Chemistry, 2014, 20, 1242–1246 CrossRef CAS PubMed.
- C. Pang, H. Cui, G. Yang and C. Wang, Nano Lett., 2013, 13, 4708–4714 CrossRef CAS PubMed.
- Y. Wang, H. Jin, Q. Ma, K. Mo, H. Mao, A. Feldhoff, X. Cao, Y. Li, F. Pan and Z. Jiang, Angew. Chem., Int. Ed., 2020, 59, 4365–4369 CrossRef CAS PubMed.
- G. Wen and Z. Guo, J. Mater. Chem. A, 2020, 8, 20238–20259 RSC.
- H. Li and Y. J. Zhu, Energy Environ. Mater., 2021, 4(4), 544–561 CrossRef.
- R. Karthick and F. Chen, Carbon, 2019, 150, 292–310 CrossRef CAS.
- S. Park, J. An, J. W. Suk and R. S. Ruoff, Small, 2010, 6, 210–212 CrossRef CAS PubMed.
- B. Simončič, S. Hadžić, J. Vasiljević, L. Černe, B. Tomšič, I. Jerman, B. Orel and J. Medved, Cellulose, 2013, 21, 595–605 CrossRef.
- L. Costes, F. Laoutid, S. Brohez and P. Dubois, Mater. Sci. Eng., R, 2017, 117, 1–25 CrossRef.
- S. Cao, P. Rathi, X. Wu, D. Ghim, Y. S. Jun and S. Singamaneni, Adv. Mater., 2021, 33, e2000922 CrossRef PubMed.
- L. Liu, Z. Niu, L. Zhang, W. Zhou, X. Chen and S. Xie, Adv. Mater., 2014, 26, 4855–4862 CrossRef CAS PubMed.
- Y. Zhang, L. Zhang, K. Cui, S. Ge, X. Cheng, M. Yan, J. Yu and H. Liu, Adv. Mater., 2018, 30, e1801588 CrossRef PubMed.
- C. H. Wang and Z. G. Guo, Nanoscale, 2020, 12, 22398–22424 RSC.
- K.-F. Liu, P.-P. Li, Y.-P. Zhang, P.-F. Liu, C.-X. Cui, J.-C. Wang, X.-J. Li and L.-B. Qu, Cellulose, 2019, 26, 3859–3872 CrossRef CAS.
- Q. Li, H. Liu, S. Zhang, D. Zhang, X. Liu, Y. He, L. Mi, J. Zhang, C. Liu, C. Shen and Z. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 21904–21914 CrossRef CAS PubMed.
- T. Arbatan, L. Zhang, X.-Y. Fang and W. Shen, Chem. Eng. J., 2012, 210, 74–79 CrossRef CAS.
- P. Khanjani, A. W. T. King, G. J. Part, L. S. Johansson, M. A. Kostiainen and R. H. A. Ras, ACS Appl. Mater. Interfaces, 2018, 10, 11280–11288 CrossRef CAS PubMed.
- C. B. Lin, H. S. Chang, Y. Zhang, F. Yang and S. Lee, Langmuir, 2021, 37, 376–384 CrossRef CAS PubMed.
- S. Zhang, W. Li, W. Wang, S. Wang and C. Qin, Appl. Surf. Sci., 2019, 497, 143816 CrossRef CAS.
- Z. Jiao, W. Chu, L. Liu, Z. Mu, B. Li, Z. Wang, Z. Liao, Y. Wang, H. Xue, S. Niu, S. Jiang, Z. Han and L. Ren, Nanoscale, 2020, 12, 8536–8545 RSC.
- A. Baidya, M. A. Ganayee, S. Jakka Ravindran, K. C. Tam, S. K. Das, R. H. A. Ras and T. Pradeep, ACS Nano, 2017, 11, 11091–11099 CrossRef CAS PubMed.
- S. Nie, H. Guo, Y. Lu, J. Zhuo, J. Mo and Z. L. Wang, Adv. Mater. Technol., 2020, 5(9), 2000454 CrossRef CAS.
- H. Li, Y. He, J. Yang, X. Wang, T. Lan and L. Peng, Carbohydr. Polym., 2019, 211, 22–30 CrossRef CAS PubMed.
- H. Wu, L. Wu, S. Lu, X. Lin, H. Xiao, X. Ouyang, S. Cao, L. Chen and L. Huang, Carbohydr. Polym., 2018, 181, 419–425 CrossRef CAS PubMed.
- K. Zhang, M. Wang, M. Wu, Q. Wu, J. Liu, J. Yang and J. Zhang, Cellulose, 2019, 27, 469–480 CrossRef.
- A. Xie, J. Cui, Y. Chen, J. Lang, C. Li, Y. Yan and J. Dai, Surf. Coat. Technol., 2019, 361, 19–26 CrossRef CAS.
- L. Jiang, Z. Tang, R. M. Clinton, V. Breedveld and D. W. Hess, ACS Appl. Mater. Interfaces, 2017, 9, 9195–9203 CrossRef CAS PubMed.
- L. Li, V. Breedveld and D. W. Hess, ACS Appl. Mater. Interfaces, 2013, 5, 5381–5386 CrossRef CAS PubMed.
- Y. Wang, Y. Liu, L. Zhang, M. Zhang, G. He and Z. Sun, Appl. Surf. Sci., 2019, 496, 143743 CrossRef CAS.
- L. Zhang, H. Kwok, X. Li and H. Z. Yu, ACS Appl. Mater. Interfaces, 2017, 9, 39728–39735 CrossRef CAS PubMed.
- B. Su, Y. Tian and L. Jiang, J. Am. Chem. Soc., 2016, 138, 1727–1748 CrossRef CAS PubMed.
- H. Teisala, M. Tuominen and J. Kuusipalo, Adv. Mater. Interfaces, 2014, 1(1), 1300026 CrossRef.
- C. Li, M. Boban, S. A. Snyder, S. P. R. Kobaku, G. Kwon, G. Mehta and A. Tuteja, Adv. Funct. Mater., 2016, 26, 6121–6131 CrossRef CAS.
- N. Lavoine, I. Desloges, A. Dufresne and J. Bras, Carbohydr. Polym., 2012, 90, 735–764 CrossRef CAS PubMed.
- T. Zhang, M. Wu, S. Kuga, C. M. Ewulonu and Y. Huang, ACS Sustainable Chem. Eng., 2020, 8, 10222–10229 CrossRef CAS.
- S. A. Jalil, M. Akram, J. A. Bhat, J. J. Hayes, S. C. Singh, M. ElKabbash and C. Guo, Appl. Surf. Sci., 2020, 506, 144952 CrossRef CAS PubMed.
- H. Ahmad, Cellulose, 2021, 28, 2715–2761 CrossRef CAS.
- M. S. Islam, N. Akter, M. M. Rahman, C. Shi, M. T. Islam, H. Zeng and M. S. Azam, ACS Sustainable Chem. Eng., 2018, 6, 9178–9188 CrossRef CAS.
- E. Amini, M. Azadfallah, M. Layeghi and R. Talaei-Hassanloui, Cellulose, 2016, 23, 557–570 CrossRef CAS.
- L. Bergamonti, M. Potenza, A. Haghighi Poshtiri, A. Lorenzi, A. M. Sanangelantoni, L. Lazzarini, P. P. Lottici and C. Graiff, Carbohydr. Polym., 2020, 231, 115773 CrossRef CAS PubMed.
- P. Tyagi, R. Mathew, C. Opperman, H. Jameel, R. Gonzalez, L. Lucia, M. Hubbe and L. Pal, Langmuir, 2019, 35, 104–112 CrossRef CAS PubMed.
- J. Zhang, Z. Guo, S. Chen, H. Dong, X. Zhang, Y. Qin, C. Yao and F. Xu, Cellulose, 2021, 28, 4371–4384 CrossRef CAS.
- M. Ghanadpour, F. Carosio, P. T. Larsson and L. Wagberg, Biomacromolecules, 2015, 16, 3399–3410 CrossRef CAS PubMed.
- J. Alongi, R. A. Carletto, F. Bosco, F. Carosio, A. Di Blasio, F. Cuttica, V. Antonucci, M. Giordano and G. Malucelli, Polym. Degrad. Stab., 2014, 99, 111–117 CrossRef CAS.
- T. Zhang, S. Kuga, M. Wu and Y. Huang, ACS Sustainable Chem. Eng., 2020, 8, 12360–12365 CrossRef CAS.
- Z. Chen, P. Xiao, J. Zhang, W. Tian, R. Jia, H. Nawaz, K. Jin and J. Zhang, Chem. Eng. J., 2020, 379, 122270 CrossRef CAS.
- Y. Pan, L. Liu and H. Zhao, Cellulose, 2018, 25, 5309–5321 CrossRef CAS.
- Z. Xue, Y. Cao, N. Liu, L. Feng and L. Jiang, J. Mater. Chem. A, 2014, 2, 2445–2460 RSC.
- Y. Si and Z. Guo, Chem. Lett., 2015, 44, 874–883 CrossRef CAS.
- M. Padaki, R. Surya Murali, M. S. Abdullah, N. Misdan, A. Moslehyani, M. A. Kassim, N. Hilal and A. F. Ismail, Desalination, 2015, 357, 197–207 CrossRef CAS.
- H. Li, H. Zhang, Y. Luo, H. Shi and L. Peng, Cellulose, 2021, 28, 5033–5053 CrossRef CAS.
- X. Li, M. Cao, H. Shan, F. Handan Tezel and B. Li, Chem. Eng. J., 2019, 358, 1101–1113 CrossRef CAS.
- Z. Xue, S. Wang, L. Lin, L. Chen, M. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270–4273 CrossRef CAS PubMed.
- J. Xi, S. Jiang, Y. Lou, H. Dai and W. Wu, Cellulose, 2021, 28, 4357–4370 CrossRef CAS.
- A. L. Ahmad, M. A. Majid and B. S. Ooi, Desalination, 2011, 268, 266–269 CrossRef CAS.
- C. Ao, J. Zhao, Q. Li, J. Zhang, B. Huang, Q. Wang, J. Gai, Z. Chen, W. Zhang and C. Lu, Carbohydr. Polym., 2020, 250, 116872 CrossRef CAS PubMed.
- L. Li, J. Zhu and Z. Zeng, ACS Appl. Mater. Interfaces, 2020, 12, 55894–55902 CrossRef CAS PubMed.
- Y. Huang, H. Zhan, D. Li, H. Tian and C. Chang, J. Membr. Sci., 2019, 591, 117362 CrossRef CAS.
- X. Xu, Y. Long, Q. Li, D. Li, D. Mao, X. Chen and Y. Chen, J. Cleaner Prod., 2019, 211, 1463–1470 CrossRef CAS.
- J. Yang, A. Xie, J. Cui, Y. Chen, J. Lang, C. Li, Y. Yan and J. Dai, Cellulose, 2020, 27, 5169–5178 CrossRef CAS.
- F. Zhang, X. Pei, K. Zhai, C. Wang, Y. Bai, B. Zhang, Y. Wang, Y. Tan, K. Xu and P. Wang, Int. J. Biol. Macromol., 2020, 162, 1118–1126 CrossRef CAS PubMed.
- M. Cheng, H. He, H. Zhu, W. Guo, W. Chen, F. Xue, S. Zhou, X. Chen and S. Wang, Carbohydr. Polym., 2019, 203, 246–255 CrossRef CAS.
- H. Zhou, X. S. Jing and Z. G. Guo, J. Colloid Interface Sci., 2021, 561, 730–740 CrossRef PubMed.
- P. Tian and Z. G. Guo, J. Bionic Eng., 2019, 16, 1–12 CrossRef.
- Q. Jiang, H. Gholami Derami, D. Ghim, S. Cao, Y.-S. Jun and S. Singamaneni, J. Mater. Chem. A, 2017, 5, 18397–18402 RSC.
- F. Jiang, T. Li, Y. Li, Y. Zhang, A. Gong, J. Dai, E. Hitz, W. Luo and L. Hu, Adv. Mater., 2018, 30, 1703453 CrossRef.
- F. Ni, P. Xiao, C. Zhang, Y. Liang, J. Gu, L. Zhang and T. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 15498–15506 CrossRef CAS PubMed.
- M. d'Halluin, J. Rull-Barrull, G. Bretel, C. Labrugère, E. Le Grognec and F.-X. Felpin, ACS Sustainable Chem. Eng., 2017, 5, 1965–1973 CrossRef.
- X. Wang, Y. He, X. Liu, G. Cheng and J. Zhu, Appl. Energy, 2017, 195, 414–425 CrossRef CAS.
- Y. Pang, J. Zhang, R. Ma, Z. Qu, E. Lee and T. Luo, ACS Energy Lett., 2020, 5, 437–456 CrossRef CAS.
- Y. Xu, D. Liu, H. Xiang, S. Ren, Z. Zhu, D. Liu, H. Xu, F. Cui and W. Wang, J. Membr. Sci., 2019, 586, 222–230 CrossRef CAS.
- C. Liu, J. Huang, C.-E. Hsiung, Y. Tian, J. Wang, Y. Han and A. Fratalocchi, Adv. Sustainable Syst., 2017, 1(1–2), 1600013 CrossRef.
- J. Wang, Y. Li, L. Deng, N. Wei, Y. Weng, S. Dong, D. Qi, J. Qiu, X. Chen and T. Wu, Adv. Mater., 2017, 29(3), 1603730 CrossRef PubMed.
- L. Zhu, T. Ding, M. Gao, C. K. N. Peh and G. W. Ho, Adv. Energy Mater., 2019, 9(22), 1900250 CrossRef.
- L. Zhang, B. Tang, J. Wu, R. Li and P. Wang, Adv. Mater., 2015, 27, 4889–4894 CrossRef CAS PubMed.
- D. Roy, M. Semsarilar, J. T. Guthrie and S. Perrier, Chem. Soc. Rev., 2009, 38, 2046–2064 RSC.
- K. Pramanik, P. Sarkar and D. Bhattacharyay, Int. J. Biol. Macromol., 2019, 122, 185–194 CrossRef CAS PubMed.
- M. C. Nongbe, G. Bretel, T. Ekou, L. Ekou, B. K. Yao, E. Le Grognec and F.-X. Felpin, Cellulose, 2018, 25, 4043–4055 CrossRef CAS.
- R. A. Crane and T. B. Scott, J. Hazard. Mater., 2012, 211–212, 112–125 CrossRef CAS PubMed.
- M. Arshadi, M. K. Abdolmaleki, H. Eskandarloo, M. Azizi and A. Abbaspourrad, ACS Sustainable Chem. Eng., 2018, 6, 11662–11676 CrossRef CAS.
- H. Tai, Z. Duan, Y. Wang, S. Wang and Y. Jiang, ACS Appl. Mater. Interfaces, 2020, 12, 31037–31053 CrossRef CAS PubMed.
- Z. Niu, W. Cheng, M. Cao, D. Wang, Q. Wang, J. Han, Y. Long and G. Han, Nano Energy, 2021, 87, 106175 CrossRef CAS.
- K.-H. Choi, J. Yoo, C. K. Lee and S.-Y. Lee, Energy Environ. Sci., 2016, 9, 2812–2821 RSC.
- Z. Weng, Y. Su, D.-W. Wang, F. Li, J. Du and H.-M. Cheng, Adv. Energy Mater., 2011, 1, 917–922 CrossRef CAS.
- L. Yuan, X. Xiao, T. Ding, J. Zhong, X. Zhang, Y. Shen, B. Hu, Y. Huang, J. Zhou and Z. L. Wang, Angew. Chem., Int. Ed., 2012, 51, 4934–4938 CrossRef CAS PubMed.
- G. Grau, R. Kitsomboonloha, S. L. Swisher, H. Kang and V. Subramanian, Adv. Funct. Mater., 2014, 24, 5067–5074 CrossRef CAS.
- V. Raghuwanshi, D. Bharti, A. K. Mahato, I. Varun and S. P. Tiwari, ACS Appl. Mater. Interfaces, 2019, 11, 8357–8364 CrossRef CAS PubMed.
- U. Zschieschang and H. Klauk, J. Mater. Chem. C, 2019, 7, 5522–5533 RSC.
- M. M. Hamedi, V. E. Campbell, P. Rothemund, F. Güder, D. C. Christodouleas, J.-F. Bloch and G. M. Whitesides, Adv. Funct. Mater., 2016, 26, 2446–2453 CrossRef CAS.
- M. Amjadi and M. Sitti, ACS Nano, 2016, 10, 10202–10210 CrossRef CAS PubMed.
- M. Weng, P. Zhou, L. Chen, L. Zhang, W. Zhang, Z. Huang, C. Liu and S. Fan, Adv. Funct. Mater., 2016, 26, 7244–7253 CrossRef CAS.
- M. Amjadi and M. Sitti, Adv. Sci., 2018, 5, 1800239 CrossRef PubMed.
- P. Sahatiya, S. S. Jones and S. Badhulika, Appl. Mater. Today, 2018, 10, 106–114 CrossRef.
- S. Cai, C. Zuo, J. Zhang, H. Liu and X. Fang, Adv. Funct. Mater., 2021, 31(20), 2100026 CrossRef CAS.
- P. Pataniya, C. K. Zankat, M. Tannarana, C. K. Sumesh, S. Narayan, G. K. Solanki, K. D. Patel, V. M. Pathak and P. K. Jha, ACS Appl. Nano Mater., 2019, 2, 2758–2766 CrossRef CAS.
- K. Pardee, A. A. Green, T. Ferrante, D. E. Cameron, A. DaleyKeyser, P. Yin and J. J. Collins, Cell, 2014, 159, 940–954 CrossRef CAS PubMed.
- J.-W. Han, B. Kim, J. Li and M. Meyyappan, J. Phys. Chem. C, 2012, 116, 22094–22097 CrossRef CAS.
- Z. Chen and C. Lu, Sens. Lett., 2005, 3, 274–295 CrossRef CAS.
- S. M. Khan, J. M. Nassar and M. M. Hussain, ACS Appl. Electron. Mater., 2020, 3, 30–52 CrossRef.
- Y. Long, P. He, R. Xu, T. Hayasaka, Z. Shao, J. Zhong and L. Lin, Carbon, 2020, 157, 594–601 CrossRef CAS.
- L. Q. Tao, K. N. Zhang, H. Tian, Y. Liu, D. Y. Wang, Y. Q. Chen, Y. Yang and T. L. Ren, ACS Nano, 2017, 11, 8790–8795 CrossRef CAS PubMed.
- X. Zheng, L. Li, K. Cui, Y. Zhang, L. Zhang, S. Ge and J. Yu, ACS Appl. Mater. Interfaces, 2018, 10, 3333–3340 CrossRef CAS PubMed.
- M. Parrilla, T. Guinovart, J. Ferre, P. Blondeau and F. J. Andrade, Adv. Healthcare Mater., 2019, 8, e1900342 CrossRef PubMed.
- H. Zhao, T. Zhang, R. Qi, J. Dai, S. Liu and T. Fei, ACS Appl. Mater. Interfaces, 2017, 9, 28002–28009 CrossRef CAS PubMed.
- R. Alrammouz, J. Podlecki, A. Vena, R. Garcia, P. Abboud, R. Habchi and B. Sorli, Sens. Actuators, B, 2019, 298, 126892 CrossRef CAS.
- S. Bi, W. Dong, B. Lan, H. Zhao, L. Hou, L. Zhu, Y. Xu and Y. Lu, Composites, Part A, 2019, 124, 105452 CrossRef CAS.
- H. Liu, H. Xiang, Y. Wang, Z. Li, L. Qian, P. Li, Y. Ma, H. Zhou and W. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 40613–40619 CrossRef CAS PubMed.
- P. Sahatiya, A. Kadu, H. Gupta, P. Thanga Gomathi and S. Badhulika, ACS Appl. Mater. Interfaces, 2018, 10, 9048–9059 CrossRef CAS PubMed.
- X. Guan, Z. Hou, K. Wu, H. Zhao, S. Liu, T. Fei and T. Zhang, Sens. Actuators, B, 2021, 339, 129879 CrossRef CAS.
- H. Liu, H. Jiang, F. Du, D. Zhang, Z. Li and H. Zhou, ACS Sustainable Chem. Eng., 2017, 5, 10538–10543 CrossRef CAS.
- Z. Zhan, R. Lin, V. T. Tran, J. An, Y. Wei, H. Du, T. Tran and W. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 37921–37928 CrossRef CAS PubMed.
- S. Li, N. Pan, Z. Zhu, R. Li, B. Li, J. Chu, G. Li, Y. Chang and T. Pan, Adv. Funct. Mater., 2019, 29(11), 1807343 CrossRef.
- B. Saha, S. Baek and J. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 4658–4666 CrossRef CAS PubMed.
- X. Liao, Z. Zhang, Q. Liao, Q. Liang, Y. Ou, M. Xu, M. Li, G. Zhang and Y. Zhang, Nanoscale, 2016, 8, 13025–13032 RSC.
- X. Liao, Q. Liao, X. Yan, Q. Liang, H. Si, M. Li, H. Wu, S. Cao and Y. Zhang, Adv. Funct. Mater., 2015, 25, 2395–2401 CrossRef CAS.
- Y. Guo, M. Zhong, Z. Fang, P. Wan and G. Yu, Nano Lett., 2019, 19, 1143–1150 CrossRef CAS PubMed.
- L. Gao, C. Zhu, L. Li, C. Zhang, J. Liu, H. D. Yu and W. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 25034–25042 CrossRef CAS PubMed.
- J. Li, B. Wang, Z. Ge, R. Cheng, L. Kang, X. Zhou, J. Zeng, J. Xu, X. Tian, W. Gao, K. Chen, C. Qiu and Z. Cheng, ACS Appl. Mater. Interfaces, 2019, 11, 39088–39099 CrossRef CAS PubMed.
- W. Wang, S. Yang, K. Ding, L. Jiao, J. Yan, W. Zhao, Y. Ma, T. Wang, B. Cheng and Y. Ni, Chem. Eng. J., 2021, 425, 129949 CrossRef CAS.
- L. Liu, Z. Jiao, J. Zhang, Y. Wang, C. Zhang, X. Meng, X. Jiang, S. Niu, Z. Han and L. Ren, ACS Appl. Mater. Interfaces, 2021, 13, 1967–1978 CrossRef CAS PubMed.
- X. Liao, Z. Zhang, Q. Liang, Q. Liao and Y. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 4151–4158 CrossRef CAS PubMed.
- Y. Li, Y. A. Samad, T. Taha, G. Cai, S.-Y. Fu and K. Liao, ACS Sustainable Chem. Eng., 2016, 4, 4288–4295 CrossRef CAS.
- H. Guo, Y. Chen, Y. Li, W. Zhou, W. Xu, L. Pang, X. Fan and S. Jiang, Composites, Part A, 2021, 143, 106309 CrossRef.
- Z. Wang, X. Fu, Z. Zhang, Y. Jiang, M. Waqar, P. Xie, K. Bi, Y. Liu, X. Yin and R. Fan, J. Cleaner Prod., 2019, 234, 588–596 CrossRef.
- D. Hu, X. Huang, S. Li and P. Jiang, Compos. Sci. Technol., 2020, 188, 107995 CrossRef CAS.
- E. Li, Y. Pan, C. Wang, C. Liu, C. Shen, C. Pan and X. Liu, Chem. Eng. J., 2021, 420, 129864 CrossRef CAS.
- L. Q. Zhang, S. G. Yang, L. Li, B. Yang, H. D. Huang, D. X. Yan, G. J. Zhong, L. Xu and Z. M. Li, ACS Appl. Mater. Interfaces, 2018, 10, 40156–40167 CrossRef CAS PubMed.
- T.-W. Lee, S.-E. Lee and Y. G. Jeong, Compos. Sci. Technol., 2016, 131, 77–87 CrossRef CAS.
- M. Imai, K. Akiyama, T. Tanaka and E. Sano, Compos. Sci. Technol., 2010, 70, 1564–1570 CrossRef CAS.
- T. W. Lee, S. E. Lee and Y. G. Jeong, ACS Appl. Mater. Interfaces, 2016, 8, 13123–13132 CrossRef CAS PubMed.
- Y. Zhan, X. Hao, L. Wang, X. Jiang, Y. Cheng, C. Wang, Y. Meng, H. Xia and Z. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 14623–14633 CrossRef CAS PubMed.
- Q. Song, F. Ye, X. Yin, W. Li, H. Li, Y. Liu, K. Li, K. Xie, X. Li, Q. Fu, L. Cheng, L. Zhang and B. Wei, Adv. Mater., 2017, 29(31), 1701583 CrossRef PubMed.
- X. Liang, T. Zhao, P. Zhu, Y. Hu, R. Sun and C. P. Wong, ACS Appl. Mater. Interfaces, 2017, 9, 40857–40867 CrossRef CAS PubMed.
- F. Xie, F. Jia, L. Zhuo, Z. Lu, L. Si, J. Huang, M. Zhang and Q. Ma, Nanoscale, 2019, 11, 23382–23391 RSC.
- T. Zhang, D. Lv, R. Liu, D. Wang, T. Li, J. Xu, Y. Xie, J. Li and L. Wang, ACS Sustainable Chem. Eng., 2021, 9, 6574–6585 CrossRef CAS.
- Q. Wang, D. Xie, J. Chen, G. Liu and M. Yu, ACS Sustainable Chem. Eng., 2020, 8, 13176–13184 CrossRef CAS.
- Y. Tian, H. Shao, X. Liu, F. Chen, Y. Li, C. Tang and Y. Zheng, ACS Appl. Mater. Interfaces, 2021, 13, 22521–22530 CrossRef CAS PubMed.
- H. Nawaz, X. Zhang, S. Chen, T. You and F. Xu, Carbohydr. Polym., 2021, 267, 118135 CrossRef CAS PubMed.
- S. Delavari, S. Ziadzade, J. Keyvan Rad, V. Hamrang and A. R. Mahdavian, Carbohydr. Polym., 2020, 247, 116756 CrossRef CAS PubMed.
- G. Bretel, E. Le Grognec, D. Jacquemin, T. Hirose, K. Matsuda and F.-X. Felpin, ACS Appl. Polym. Mater., 2019, 1, 1240–1250 CrossRef CAS.
- H. Chen, X. Yan, Q. Feng, P. Zhao, X. Xu, D. H. L. Ng and L. Bian, ACS Sustainable Chem. Eng., 2017, 5, 11387–11394 CrossRef CAS.
- J. Rull-Barrull, M. d'Halluin, E. Le Grognec and F. X. Felpin, Chem. Commun., 2016, 52, 2525–2528 RSC.
- W. Lu, J. Zhang, Y. Huang, P. Theato, Q. Huang and T. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 23884–23893 CrossRef CAS PubMed.
- H. Wang, S. I. Vagin, B. Rieger and A. Meldrum, ACS Appl. Mater. Interfaces, 2020, 12, 20507–20513 CrossRef CAS PubMed.
- M. Schwanninger, J. C. Rodrigues, H. Pereira and B. Hinterstoisser, Vib. Spectrosc., 2004, 36, 23–40 CrossRef CAS.
- M. Ibrahim, M. Labaki, J. M. Giraudon and J. F. Lamonier, J. Hazard. Mater., 2020, 383, 121139 CrossRef CAS PubMed.
- Y.-Y. Jiang, Y.-J. Zhu, F. Chen and J. Wu, Ceram. Int., 2015, 41, 6098–6102 CrossRef CAS.
- M. Sadat-Shojai, M. T. Khorasani, E. Dinpanah-Khoshdargi and A. Jamshidi, Acta Biomater., 2013, 9, 7591–7621 CrossRef CAS PubMed.
- Z. C. Xiong, Z. Y. Yang, Y. J. Zhu, F. F. Chen, Y. G. Zhang and R. L. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 22212–22222 CrossRef CAS PubMed.
- L.-Y. Dong and Y.-J. Zhu, ACS Sustainable Chem. Eng., 2018, 6, 17239–17251 CrossRef CAS.
- F. F. Chen, Y. J. Zhu, Z. C. Xiong, L. Y. Dong, F. Chen, B. Q. Lu and R. L. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 39534–39548 CrossRef CAS PubMed.
- F. F. Chen, Y. J. Zhu, F. Chen, L. Y. Dong, R. L. Yang and Z. C. Xiong, ACS Nano, 2018, 12, 3159–3171 CrossRef CAS PubMed.
- H. Li, Y.-J. Zhu, Y.-Y. Jiang, Y.-D. Yu, F. Chen, L.-Y. Dong and J. Wu, ChemNanoMat, 2017, 3, 259–268 CrossRef CAS.
- L.-Y. Dong and Y.-J. Zhu, ACS Sustainable Chem. Eng., 2020, 8, 17500–17507 CrossRef CAS.
- F. F. Chen, Y. J. Zhu, Z. C. Xiong, T. W. Sun and Y. Q. Shen, ACS Appl. Mater. Interfaces, 2016, 8, 34715–34724 CrossRef CAS PubMed.
- L. Cheng and J. Feng, Chem. Eng. J., 2020, 398, 125633 CrossRef CAS.
- C. Shen, Y. Zhu, X. Xiao, X. Xu, X. Chen and G. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 35142–35151 CrossRef CAS PubMed.
- Y. Lin, H. Xu, X. Shan, Y. Di, A. Zhao, Y. Hu and Z. Gan, J. Mater. Chem. A, 2019, 7, 19203–19227 RSC.
- N. S. Fuzil, N. H. Othman, N. H. Alias, F. Marpani, M. H. D. Othman, A. F. Ismail, W. J. Lau, K. Li, T. D. Kusworo, I. Ichinose and M. M. A. Shirazi, Desalination, 2021, 517, 115259 CrossRef CAS.
- Z. C. Xiong, Y. J. Zhu, D. D. Qin, F. F. Chen and R. L. Yang, Small, 2018, 14, e1803387 CrossRef PubMed.
- Z. Li, X. Xu, X. Sheng, P. Lin, J. Tang, L. Pan, Y. V. Kaneti, T. Yang and Y. Yamauchi, ACS Nano, 2021, 15(8), 12535–12566 CrossRef CAS PubMed.
- S. Cao, X. Wu, Y. Zhu, R. Gupta, A. Tan, Z. Wang, Y.-S. Jun and S. Singamaneni, J. Mater. Chem. A, 2020, 8, 5147–5156 RSC.
- Z. C. Xiong, Y. J. Zhu, D. D. Qin and R. L. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 32556–32565 CrossRef CAS PubMed.
- R.-L. Yang, Y.-J. Zhu, F.-F. Chen, D.-D. Qin and Z.-C. Xiong, ACS Sustainable Chem. Eng., 2019, 7, 13226–13235 CrossRef CAS.
- J. Chen, J. L. Yin, B. Li, Z. Ye, D. Liu, D. Ding, F. Qian, N. V. Myung, Q. Zhang and Y. Yin, ACS Nano, 2020, 14(12), 17419–17427 CrossRef CAS.
- D. D. Qin, Y. J. Zhu, R. L. Yang and Z. C. Xiong, Nanoscale, 2020, 12, 6717–6728 RSC.
- T. A. Cooper, S. H. Zandavi, G. W. Ni, Y. Tsurimaki, Y. Huang, S. V. Boriskina and G. Chen, Nat. Commun., 2018, 9, 5086 CrossRef CAS PubMed.
- R. L. Yang, Y. J. Zhu, D. D. Qin and Z. C. Xiong, ACS Appl. Mater. Interfaces, 2020, 12, 1339–1347 CrossRef CAS PubMed.
- G. Wen and Z. Guo, J. Mater. Chem. A, 2018, 6, 7042–7052 RSC.
- G. Wen, X. Gao, P. Tian, L. Zhong, Z. Wang and Z. Guo, Chem. Eng. J., 2018, 346, 94–103 CrossRef CAS.
- R.-L. Yang, Y.-J. Zhu, F.-F. Chen, D.-D. Qin and Z.-C. Xiong, ACS Sustainable Chem. Eng., 2018, 6, 10140–10150 CrossRef CAS.
- Z.-C. Xiong, Z.-Y. Yang, Y.-J. Zhu, F.-F. Chen, R.-L. Yang and D.-D. Qin, J. Mater. Chem. A, 2018, 6, 5762–5773 RSC.
- Y. Li, H. Zhou, G. Zhu, C. Shao, H. Pan, X. Xu and R. Tang, J. Hazard. Mater., 2015, 299, 379–387 CrossRef CAS PubMed.
- F. F. Chen, Y. J. Zhu, Z. C. Xiong, T. W. Sun, Y. Q. Shen and R. L. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 11045–11053 CrossRef CAS PubMed.
- J. He, Y. Li, X. Cai, K. Chen, H. Zheng, C. Wang, K. Zhang, D. Lin, L. Kong and J. Liu, Chemosphere, 2017, 174, 380–389 CrossRef CAS PubMed.
- Y. You, K. Qu, Z. Huang, R. Ma, C. Shi, X. Li, D. Liu, M. Dong and Z. Guo, Int. J. Biol. Macromol., 2019, 141, 1035–1043 CrossRef CAS PubMed.
- Q. Q. Zhang, Y. J. Zhu, J. Wu, Y. T. Shao, A. Y. Cai and L. Y. Dong, ACS Appl. Mater. Interfaces, 2019, 11, 4288–4301 CrossRef CAS PubMed.
- R. L. Yang, Y. J. Zhu, F. F. Chen, L. Y. Dong and Z. C. Xiong, ACS Appl. Mater. Interfaces, 2017, 9, 25455–25464 CrossRef CAS PubMed.
- Z.-C. Xiong, R.-L. Yang, Y.-J. Zhu, F.-F. Chen and L.-Y. Dong, J. Mater. Chem. A, 2017, 5, 17482–17491 RSC.
- Z. C. Xiong, Y. J. Zhu, F. F. Chen, T. W. Sun and Y. Q. Shen, Chemistry, 2016, 22, 11224–11231 CrossRef CAS PubMed.
- G. Wen, X. Gao and Z. Guo, J. Mater. Chem. A, 2018, 6, 21524–21531 RSC.
- F. Xu, R. Chen, Z. Lin, X. Sun, S. Wang, W. Yin, Q. Peng, Y. Li and X. He, J. Mater. Chem. C, 2018, 6, 12321–12328 RSC.
- H. Zhang, D. Yang, A. Lau, T. Ma, H. Lin and B. Jia, Small, 2021, 17, e2007311 CrossRef PubMed.
- S. P. Meryl, D. Stoller, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef PubMed.
- F. C. R. Karthick, Carbon, 2019, 150, 292–310 CrossRef.
- C. Wang, D. Li, C. O. Too and G. G. Wallace, Chem. Mater., 2009, 21, 2604–2606 CrossRef CAS.
- F. Liu, S. Song, D. Xue and H. Zhang, Adv. Mater., 2012, 24, 1089–1094 CrossRef CAS PubMed.
- P. Kumar, F. Shahzad, S. Yu, S. M. Hong, Y.-H. Kim and C. M. Koo, Carbon, 2015, 94, 494–500 CrossRef CAS.
- P. Liu, H. Yang, X. Zhang, M. Jiang, Y. Duan and J. Zhang, Carbon, 2016, 107, 46–55 CrossRef CAS.
- C. Teng, D. Xie, J. Wang, Z. Yang, G. Ren and Y. Zhu, Adv. Funct. Mater., 2017, 27(20), 1700240 CrossRef.
- O. Sadak, A. K. Sundramoorthy and S. Gunasekaran, Carbon, 2018, 138, 108–117 CrossRef CAS.
- B. Shen, W. Zhai and W. Zheng, Adv. Funct. Mater., 2014, 24, 4542–4548 CrossRef CAS.
- G. Xin, H. Sun, T. Hu, H. R. Fard, X. Sun, N. Koratkar, T. Borca-Tasciuc and J. Lian, Adv. Mater., 2014, 26, 4521–4526 CrossRef CAS PubMed.
- A. A. Balandin, Nat. Mater., 2011, 10, 569–581 CrossRef CAS PubMed.
- J. Gao, Q. Yan, L. Lv, X. Tan, J. Ying, K. Yang, J. Yu, S. Du, Q. Wei, R. Xiang, Y. Yao, X. Zeng, R. Sun, C.-P. Wong, N. Jiang, C.-T. Lin and W. Dai, Chem. Eng. J., 2021, 419, 129609 CrossRef CAS.
- Z. Qu, K. Wu, C.-a. Xu, Y. Li, E. Jiao, B. Chen, H. Meng, X. Cui, K. Wang and J. Shi, Chem. Mater., 2021, 33, 3228–3240 CrossRef CAS.
- Z. Shen and J. Feng, ACS Appl. Nano Mater., 2017, 1, 94–100 CrossRef.
- A. Giri and P. E. Hopkins, Adv. Funct. Mater., 2019, 30, 1903857 CrossRef.
- S. Hong, S. S. Yoo and P. J. Yoo, J. Mater. Chem. C, 2019, 7, 9380–9388 RSC.
- Q.-Q. Kong, Z. Liu, J.-G. Gao, C.-M. Chen, Q. Zhang, G. Zhou, Z.-C. Tao, X.-H. Zhang, M.-Z. Wang, F. Li and R. Cai, Adv. Funct. Mater., 2014, 24, 4222–4228 CrossRef CAS.
- Y. Chen, X. Hou, R. Kang, Y. Liang, L. Guo, W. Dai, K. Nishimura, C.-T. Lin, N. Jiang and J. Yu, J. Mater. Chem. C, 2018, 6, 12739–12745 RSC.
- A. C. Ferrari and D. M. Basko, Nat. Nanotechnol., 2013, 8, 235–246 CrossRef CAS PubMed.
- Y. Li, W. Wei, Y. Wang, N. Kadhim, Y. Mei and Z. Zhou, J. Mater. Chem. C, 2019, 7, 11783–11789 RSC.
- B. Nan, K. Wu, Z. Qu, L. Xiao, C. Xu, J. Shi and M. Lu, Carbon, 2020, 161, 132–145 CrossRef CAS.
- L. Peng, Z. Xu, Z. Liu, Y. Guo, P. Li and C. Gao, Adv. Mater., 2017, 29(27), 1700589 CrossRef PubMed.
- Y. Liu, K. Wu, M. Lu, E. Jiao, H. Zhang, J. Shi and M. Lu, Composites, Part A, 2021, 141, 106227 CrossRef CAS.
- X. Meng, H. Pan, C. Zhu, Z. Chen, T. Lu, D. Xu, Y. Li and S. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 22611–22622 CrossRef CAS PubMed.
- Y. Yao, X. Zeng, F. Wang, R. Sun, J.-b. Xu and C.-P. Wong, Chem. Mater., 2016, 28, 1049–1057 CrossRef CAS.
- D. An, S. Cheng, S. Xi, Z. Zhang, X. Duan, Y. Ren, J. Li, Z. Sun, Y. Liu and C.-P. Wong, Chem. Eng. J., 2020, 383, 123151 CrossRef CAS.
- Z. Qu, K. Wang, C.-a. Xu, Y. Li, E. Jiao, B. Chen, H. Meng, X. Cui, J. Shi and K. Wu, Chem. Eng. J., 2021, 421, 129729 CrossRef CAS.
- Y. Shao, M. F. El-Kady, C. W. Lin, G. Zhu, K. L. Marsh, J. Y. Hwang, Q. Zhang, Y. Li, H. Wang and R. B. Kaner, Adv. Mater., 2016, 28, 6719–6726 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7(11), 845–854 CrossRef CAS PubMed.
- L. Huang, D. Santiago, P. Loyselle and L. Dai, Small, 2018, 14, e1800879 CrossRef PubMed.
- D.-Y. Wu, W.-H. Zhou, L.-Y. He, H.-Y. Tang, X.-H. Xu, Q.-S. Ouyang and J.-J. Shao, Carbon, 2020, 160, 156–163 CrossRef CAS.
- L. Li, B. Song, L. Maurer, Z. Lin, G. Lian, C.-C. Tuan, K.-S. Moon and C.-P. Wong, Nano Energy, 2016, 21, 276–294 CrossRef CAS.
- L. Jiang, L. Sheng, C. Long and Z. Fan, Nano Energy, 2015, 11, 471–480 CrossRef CAS.
- A. Abdollahi, A. Abnavi, S. Ghasemi, S. Mohajerzadeh and Z. Sanaee, Electrochim. Acta, 2019, 320, 134598 CrossRef CAS.
- D. Wu, C. Yu and W. Zhong, J. Mater. Chem. A, 2021, 9, 18356–18368 RSC.
- L. Deng, Y. Gu, Y. Gao, Z. Ma and G. Fan, J. Colloid Interface Sci., 2017, 494, 355–362 CrossRef CAS PubMed.
- L. Huang, C. Li and G. Shi, J. Mater. Chem. A, 2014, 2, 968–974 RSC.
- X. Yang, J. Zhu, L. Qiu and D. Li, Adv. Mater., 2011, 23, 2833–2838 CrossRef CAS PubMed.
- G. Lian, C. C. Tuan, L. Li, S. Jiao, K. S. Moon, Q. Wang, D. Cui and C. P. Wong, Nano Lett., 2017, 17, 1365–1370 CrossRef CAS PubMed.
- S. Wang, X. Wang, C. Sun and Z.-S. Wu, J. Energy Chem., 2021, 61, 23–28 CrossRef.
- J. Cao, C. Chen, K. Chen, Q. Lu, Q. Wang, P. Zhou, D. Liu, L. Song, Z. Niu and J. Chen, J. Mater. Chem. A, 2017, 5, 15008–15016 RSC.
- Y. Xiong, M. Zhou, H. Chen, L. Feng, Z. Wang, X. Yan and S. Guan, Appl. Surf. Sci., 2015, 357, 1024–1030 CrossRef CAS.
- S. Yue, H. Tong, L. Lu, W. Tang, W. Bai, F. Jin, Q. Han, J. He, J. Liu and X. Zhang, J. Mater. Chem. A, 2017, 5, 689–698 RSC.
- Y. Xu, X. Chen, C. Huang, Y. Zhou, B. Fan, Y. Li, A. Hu, Q. Tang and K. Huang, J. Power Sources, 2021, 488, 229426 CrossRef CAS.
- K. Shu, Y. Chao, S. Chou, C. Wang, T. Zheng, S. Gambhir and G. G. Wallace, ACS Appl. Mater. Interfaces, 2018, 10, 22031–22041 CrossRef CAS PubMed.
- C. Huang, Q. Tang, Q. Feng, Y. Li, Y. Xu, Y. Zhang, A. Hu, S. Zhang, W. Deng and X. Chen, J. Mater. Chem. A, 2020, 8, 9661–9669 RSC.
- H. W. Dandan Yu, J. Yang, Z. Q. Niu, H. T. Lu, Y. Yang, L. W. Cheng and L. Guo, ACS Appl. Mater. Interfaces, 2017, 9(25), 21298–21306 CrossRef PubMed.
- Y. Zhao, J. Liu, B. Wang, J. Sha, Y. Li, D. Zheng, M. Amjadipour, J. MacLeod and N. Motta, ACS Appl. Mater. Interfaces, 2017, 9, 22588–22596 CrossRef CAS PubMed.
- C. Huang, A. Hu, Y. Li, H. Zhou, Y. Xu, Y. Zhang, S. Zhou, Q. Tang, C. Chen and X. Chen, Nanoscale, 2019, 11, 16515–16522 RSC.
- X. Yu, L. Feng and H. S. Park, J. Power Sources, 2018, 390, 93–99 CrossRef CAS.
- Q. Zhou, W. Ju, Y. Yong, Q. Zhang, Y. Liu and J. Li, Carbon, 2020, 170, 368–379 CrossRef CAS.
- B. Yuan, Y. Wang, G. Chen, F. Yang, H. Zhang, C. Cao and B. Zuo, J. Hazard. Mater., 2021, 403, 123645 CrossRef CAS PubMed.
- G. Chen, B. Yuan, Y. Wang, X. Chen, C. Huang, S. Shang, H. Tao, J. Liu, W. Sun, P. Yang and G. Shi, J. Colloid Interface Sci., 2020, 578, 412–421 CrossRef CAS PubMed.
- Z. Qu, C.-a. Xu, X. Li, Y. Wu, K. Wang, X. Zheng, X. Cui, X. Wu, J. Shi and K. Wu, Chem. Eng. J., 2021, 426, 130717 CrossRef CAS.
- L. Dong, C. Hu, L. Song, X. Huang, N. Chen and L. Qu, Adv. Funct. Mater., 2016, 26, 1470–1476 CrossRef CAS.
- N.-J. Huang, C.-F. Cao, Y. Li, L. Zhao, G.-D. Zhang, J.-F. Gao, L.-Z. Guan, J.-X. Jiang and L.-C. Tang, Composites, Part B, 2019, 168, 413–420 CrossRef CAS.
- I.-Y. J. M.-J. Kim, J.-M. Seo, L. Dai and J.-B. Baek, ACS Nano, 2014, 8, 2820–2825 CrossRef PubMed.
- N.-J. Huang, Q.-Q. Xia, Z.-H. Zhang, L. Zhao, G.-D. Zhang, J.-F. Gao and L.-C. Tang, Composites, Part A, 2020, 131, 105797 CrossRef CAS.
- Z.-H. Zhang, J.-W. Zhang, C.-F. Cao, K.-Y. Guo, L. Zhao, G.-D. Zhang, J.-F. Gao and L.-C. Tang, Chem. Eng. J., 2020, 386, 123894 CrossRef CAS.
- Z.-R. Yu, S.-N. Li, J. Zang, M. Zhang, L.-X. Gong, P. Song, L. Zhao, G.-D. Zhang and L.-C. Tang, Composites, Part B, 2019, 177, 107347 CrossRef CAS.
- W. Wang, C. Xiang, Q. Zhu, W. Zhong, M. Li, K. Yan and D. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 27215–27223 CrossRef CAS PubMed.
- Y. L. Zhang, Y. Q. Liu, D. D. Han, J. N. Ma, D. Wang, X. B. Li and H. B. Sun, Adv. Mater., 2019, 31, e1901585 CrossRef PubMed.
- Y. Hu, J. Liu, L. Chang, L. Yang, A. Xu, K. Qi, P. Lu, G. Wu, W. Chen and Y. Wu, Adv. Funct. Mater., 2017, 27(44), 1704388 CrossRef.
- B. Han, Y.-L. Zhang, Q.-D. Chen and H.-B. Sun, Adv. Funct. Mater., 2018, 28(40), 1802235 CrossRef.
- T. Xu, Q. Han, Z. Cheng, J. Zhang and L. Qu, Small Methods, 2018, 2, 1800108 CrossRef.
- D. D. Han, Y. L. Zhang, H. B. Jiang, H. Xia, J. Feng, Q. D. Chen, H. L. Xu and H. B. Sun, Adv. Mater., 2015, 27, 332–338 CrossRef CAS PubMed.
- L. Guo, H.-B. Jiang, R.-Q. Shao, Y.-L. Zhang, S.-Y. Xie, J.-N. Wang, X.-B. Li, F. Jiang, Q.-D. Chen, T. Zhang and H.-B. Sun, Carbon, 2012, 50, 1667–1673 CrossRef CAS.
- H. B. Jiang, Y. Liu, J. Liu, S. Y. Li, Y. Y. Song, D. D. Han and L. Q. Ren, Front. Chem., 2019, 7, 464 CrossRef CAS PubMed.
- J. N. Ma, J. W. Mao, D. D. Han, X. Y. Fu, Y. X. Wang and Y. L. Zhang, Adv. Mater. Technol., 2019, 4(11), 1900554 CrossRef CAS.
- J. Mu, C. Hou, B. Zhu, H. Wang, Y. Li and Q. Zhang, Sci. Rep., 2015, 5, 9503 CrossRef CAS PubMed.
- D.-D. Han, Y.-L. Zhang, Y. Liu, Y.-Q. Liu, H.-B. Jiang, B. Han, X.-Y. Fu, H. Ding, H.-L. Xu and H.-B. Sun, Adv. Funct. Mater., 2015, 25, 4548–4557 CrossRef CAS.
- C. Y. H. J. K. Mu, H. Z. Wang, Y. G. Li, Q. H. Zhang and M. F. Zhu, Sci. Adv., 2015, 1, e1500533 CrossRef PubMed.
- Y. Jiang, C. Hu, H. Cheng, C. Li, T. Xu, Y. Zhao, H. Shao and L. Qu, ACS Nano, 2016, 10, 4735–4741 CrossRef CAS PubMed.
- H. Cheng, J. Liu, Y. Zhao, C. Hu, Z. Zhang, N. Chen, L. Jiang and L. Qu, Angew. Chem., Int. Ed., 2013, 52, 10482–10486 CrossRef CAS PubMed.
- L. Chen, M. Weng, P. Zhou, L. Zhang, Z. Huang and W. Zhang, Nanoscale, 2017, 9, 9825–9833 RSC.
- H. Cheng, F. Zhao, J. Xue, G. Shi, L. Jiang and L. Qu, ACS Nano, 2016, 10, 9529–9535 CrossRef CAS PubMed.
- P. Zhu, Y. Kuang, Y. Wei, F. Li, H. Ou, F. Jiang and G. Chen, Chem. Eng. J., 2021, 404, 127105 CrossRef CAS PubMed.
- A. B. Dichiara, A. Song, S. M. Goodman, D. He and J. Bai, J. Mater. Chem. A, 2017, 5, 20161–20169 RSC.
- J. Xing, P. Tao, Z. Wu, C. Xing, X. Liao and S. Nie, Carbohydr. Polym., 2019, 207, 447–459 CrossRef CAS PubMed.
- Q.-Q. Zhang, Y.-J. Zhu, J. Wu and L.-Y. Dong, ACS Sustainable Chem. Eng., 2019, 7, 17198–17209 CrossRef CAS.
- Q. Q. Zhang, Y. J. Zhu, J. Wu, Y. T. Shao and L. Y. Dong, J. Colloid Interface Sci., 2020, 575, 78–87 CrossRef CAS PubMed.
- F. F. Chen, Z. H. Dai, Y. N. Feng, Z. C. Xiong, Y. J. Zhu and Y. Yu, ACS Nano, 2021, 15, 5355–5365 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |


