Biomass into chemicals: green chemical conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids
Amutha Chinnappan
a,
Chinnappan Baskar
b and
Hern Kim
*a
aDepartment of Energy Science and Technology, Smart Living Innovation Technology Center, Myongji University, Yongin, Gyeonggi-do 17058, Republic of Korea. E-mail: hernkim@mju.ac.kr; Fax: +82 31 336 6336; Tel: +82 31 330 6688
bTHDC Institute of Hydropower Engineering and Technology Tehri, Uttarakhand Technical University, Dehradun, Uttarakhand, India 249001
First published on 29th June 2016
Abstract
Biomass is one of the few resources that have the potential to meet the challenges of sustainable and green energy systems. Already numerous valuable chemicals are derived from renewable resources; among these, 5-hydroxymethylfurfural (HMF), plays a vital role, because it can be obtained from carbohydrates. HMF is a versatile platform chemical for the synthesis of a wide range of industrially important materials, including biofuels. HMF is the key intermediate to bridge the gap between biomass resources and biochemicals. Recent studies have shown that all of the components of biomass are soluble in ionic liquids. Ionic liquids have undergone a remarkable process of evolution for carbohydrate conversion and several new ionic liquids have been reported. This review mainly focuses on the production of HMF from carbohydrates by using ionic liquids as solvents as well as catalysts and their reuse.
Introduction
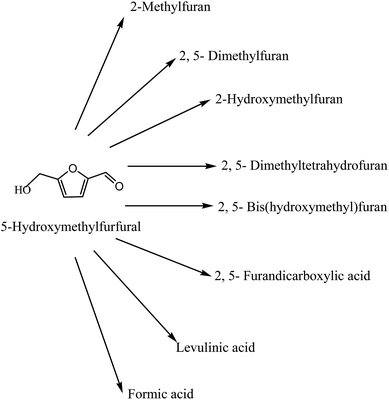
Today's energy system is unsustainable because of equity issues as well as environmental, economic, and geopolitical concerns that have implications far into the future. At present, fossil fuels are playing the major role to meet the energy requirements of the world. The large consumption of fossil fuels creates so many environmental problems, such as greenhouse gases, which has motivated the search for alternative, renewable fuel options.1–5 Biomass provides a clean way to dramatically improve our environment, economy and energy security. All organic matter is known as biomass, and the energy released from biomass when it is eaten, burnt or converted into fuels is called biomass energy (Fig. 1). Biomass energy generates far less air emissions than fossil fuels.6–8 Renewable energy is one of the most efficient ways to achieve sustainable development. Biomass has been used to meet the needs of civilization since prehistory. Several biomass chemical products can be traced to a time when neither the chemical mechanistic transformation process nor the chemical identities of the product were understood. These early products include chemicals still widely used, such as ethanol and acetic. A more scientific understanding of chemistry in the 19th century led to the use of biomass as a feedstock for chemical processes.9–12Current biomass resources comprise primarily industrial waste materials such as sawdust or pulp process wastes, hog fuel, forest residues, clean wood waste from landfills, and agricultural prunings and residues from plants such as lignocellulosic materials.13–22 There are already a considerable range of chemical building blocks derived from renewable resources. One of these, 5-hydroxymethylfurfural (HMF), plays an important role, because it is versatile and key intermediate to the production of high value polymers such as polyurethanes and polyamides, as well as for biofuels such as dimethylfuran (DMF) and other molecules, but also for important molecules such as levulinic acid, 2,5-furandicarboxylic acid (FDA), 2,5-diformylfuran (DFF), dihydroxymethylfuran and 5-hydroxy-4-keto-2-pentenoic acid (Fig. 2).14,23–28 Moreover they can be obtained not only from fructose but also from glucose via isomerisation to fructose, as well as directly from cellulose.
The dehydration of simple sugars to form HMF has been conducted in water and in various organic solvents such as dimethyl sulfoxide, dimethylformamide, and dimethylacetamide in presence of different acidic catalysts.29–31 Strongly acidic ion-exchange resin,32 zeolites33 supported heteropolyacid,33,34 and inorganic salts have also been employed for the dehydration of fructose.35,36 Many of these reactions require drastic reaction conditions, excess of solvent and catalysts or reagents, which limits the use of large scale reaction for above methods. HMF production is currently still facing significant technical challenges to make it economically feasible in an industrial scale. A simple and an efficient method to produce pure HMF from abundant renewable carbohydrates in high yield at low energy cost must be developed before a biorefinery platform can be built on the basis of this substrate.14,37,38 Sugar molecules are potential feedstock for this purpose. Ionic liquids (ILs) can be a good choice as solvents and catalysts for conversion of sugars into HMF and these processes have received more attention.14,39–43
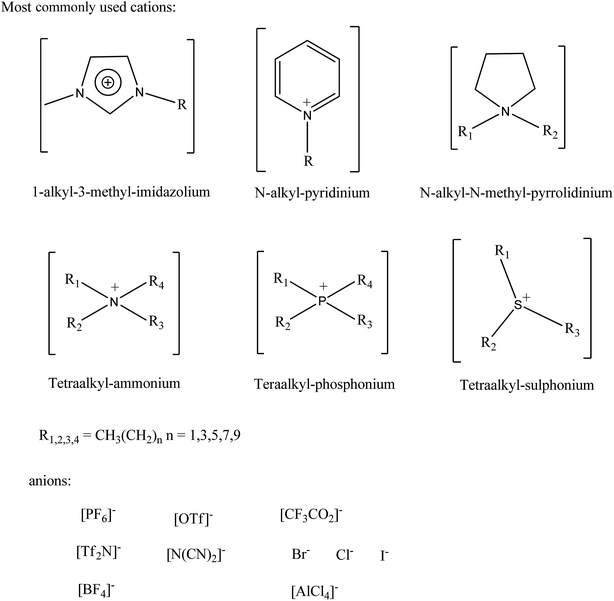
The attraction of ionic liquids lies in their remarkable set of properties when compared to conventional solvents. As salts consisting of distinct anions and cations, ILs are inherently binary (or higher order) systems. The anions and cations can be independently selected to tune the IL's physicochemical properties (melting point, conductivity, viscosity, density, refractive index, etc.) while at the same time introducing specific features for a given application (hydrophobicity vs. hydrophilicity, controlling solute solubility, adding functional groups for catalysis/reactivity purposes, chirality, etc.). Examples of typical IL anions and cations are shown in Fig. 3, however these are only a sample of the infinite variety available.44–48
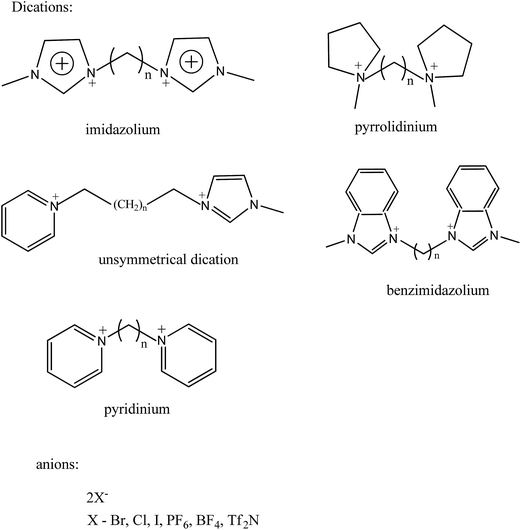
Generally, their synthesis includes two steps, the first to obtain the wanted cation by protonation or alkylation and the second to get the desired anion by either directly treating with Lewis acids or exchanging anions. Anion exchange can be further divided into the anion metathesis method, with metal salts or protic acids, and the anion exchange resin method.49–51 Fig. 4 shows the general method for synthesis of ionic liquids. Same principles are followed for the dicationic ionic liquids also. Firstly, the deprotonation of desired cation followed by addition of two equivalents of the functionalized alkyl halide precursor usually gives high yield of dicationic ILs.52 Dicationic ILs have been classified as geminal (the two monocations that form the dication are the same, for example, imidazolium–R–imidazolium where R is the alkyl linker) or unsymmetrical53 (the mono-cations are different, for example, imidazolium–R–pyridinium). While dicationic ILs in which each dication is associated with two monoanions such as PF6− and/or Tf2N− have been intensively reported (Fig. 5).54–56
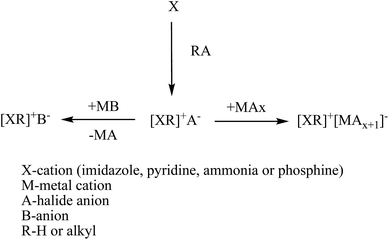 | ||
| Fig. 4 Typical schematic representation for synthesis of ionic liquids.50 | ||
HMF synthesis
This review paper mainly focus on the production of HMF from carbohydrates by using ionic liquids as solvents as well as catalyst and their reuse. In addition, the mechanisms of the different synthetic route are discussed. In recent years, ionic liquids have undergone a remarkable process of evolution for carbohydrates conversion and several new ionic liquids have been reported (Table 1).| Feedstock | Ionic liquid | Solvent/catalyst | Temp. (°C) | Time | Con (%) | Sele. (%) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| a Con, conversion; Sele., selectivity; [C6(mpy)2]Br2−, 1,1′-decane-1,10-diylbis(3-methylpyridinium)dibromide; [C6(mpy)2][NiCl4]2−, 1,1′-hexane-1,6-diylbis(3-methylpyridinium)tetrachloronickelate(II); [C6(mpy)2][CoCl4]2−, 1,1′-hexane-1,6-diylbis(3-methylpyridinium)tetrachlorocobaltate(II); [C10(Epy)2]2Br−, 1,1′-decane-1,10-diylbis(3-ethylpyridinium)dibromide; [C4MIM]Cl, 1-butyl-3-methylimidazolium chloride; [BMIM]Cl, 1-butyl-3-methylimidazolium chloride; [CMIm]Cl, 1-carboxypropyl-3-methyl imidazolium chloride; [tetraEG(mim)(triethylamo)](HSO4)2, [1(3-methylimidazoliumtetraethoxy)(triethylammonium)][bis(hydrogen sulfate)]; [tetraEG(mim)2][OMs]2−, tetra ethylene glycol-bis(3-methylimidazolium)dimesylate; [AMIM]Cl, 1-allyl-3-methylimidazolium chloride; [BMIM][BF4], 1-butyl-3-methylimidazolium tetrafluoroborate; [BMIM]OH, 1-butyl-3-methylimidazolium hydroxide; [C4C1im][HSO4], 1-butyl-3-methylimidazolium hydrogen sulfate; P[BVIM]Cl, poly(3-butyl-1-vinylimidazolium chloride); [OMIM][Cl], 1-methyl-3-octylimidazolium chloride; [BMIM]Br, 1-butyl-3-methylimidazolium bromide; [EMIM]Br, 1-ethyl-3-methylimidazolium bromide; DMSO, dimethylsulfoxide; GeCl4, germanium tetrachloride; EtOH, ethanol; [HNMP][CH3SO3], N-methyl-2-pyrrolidonium methyl sulfonate; IrCl3, iridium(III)chloride; NMP, N-methyl-2-pyrrolidonium; MIBK, methyl isobutyl ketone; CrCl3·6H2O, chromium(III)chloride hexahydrate; CrCl2, chromium(II)chloride; Cr(NO3)3, chromium nitrate; Zr(NO3)4, zirconium(IV)hydroxide; AuCl3·HCl, gold(III)chloride, hydrogen chloride; Ce(NH4)2(NO3)6, ceric ammonium nitrate; H3PW12O40, phosphotungstic acid; H3PMoO40, phosphomolybdic acid. | ||||||||
| Fructose | [C6(mpy)2]Br2− | DMSO | 110 | 60 min | 95.2 | 95.5 | 91.0 | 39 |
| Fructose | [C6(mpy)2][NiCl4]2− | DMSO | 110 | 60 min | 95.6 | 99.8 | 95.5 | 39 |
| Fructose | [C6(mpy)2][CoCl4]2− | DMSO | 110 | 60 min | 95.9 | 81.2 | 77.9 | 39 |
| Fructose | [C10(Epy)2]2Br− | DMSO | 110 | 60 min | 96.0 | 86.1 | 83.0 | 39 |
| Fructose | [C10(Epy)2]2Br− | — | 100 | 90 min | 89.2 | 97.7 | 87.1 | 39 |
| Fructose | [C4MIM]Cl | — | 155 | 5 min | 84 | — | 82 | 107 |
| Fructose | [BMIM]Cl | DMSO/GeCl4 | 25 | 12 h | — | — | 42.9 | 68 |
| Fructose | [BMIM]Cl | EtOH/[HNMP][CH3SO3] | 25 | 3 h | — | — | 78.4 | 71 |
| Fructose | [CMIm]Cl | DMSO | 120 | 120 min | 95.7 | 62 | ||
| Fructose | ([TetraEG(mim)(triethylamo)])(HSO4−)2 | — | 70 | 40 min | 100 | — | 92.3 | 81 |
| Fructose | [TetraEG(mim)2][OMs]2− | — | 120 | 40 min | — | — | 92.3 | 82 |
| Fructose | [AMIM]Cl | DMF | 100 | 45 min | — | — | 84.9 | 108 |
| Fructose | [BMIM][BF4]/[BMIM][Cl] | Amberlyst 15 | 25 | 3 h | — | — | 56 | 109 |
| Fructose | [BMIM]Cl | IrCl3 | 120 | 30 min | — | — | 97.7 | 110 |
| Fructose | [BMIM]OH | DMSO | 160 | 120 min | 58.9 | 54.7 | 92.9 | 111 |
| Fructose | [BMIM]OH | NMP | 160 | 120 min | 14.1 | 3.7 | 26.2 | 111 |
| Fructose | [BMIM]OH | MIBK | 160 | 120 min | 7.9 | 2.7 | 34.2 | 111 |
| Fructose | [BMIM]OH | n-Butyl alcohol | 160 | 120 min | 2.7 | 0.6 | 22.2 | 111 |
| Fructose | [BMIM]OH | sec-Butylalcohol | 160 | 120 min | 14.6 | 1.5 | 10.3 | 111 |
| Fructose | [BMIM]OH | Isobutyl alcohol | 160 | 120 min | 3.1 | 0.5 | 16.1 | 111 |
| Fructose | [BMIM]Cl | D001-cc resin | 75 | 20 min | — | — | 93 | 63 |
| Fructose | [AMIM]Cl | DMF | 100 | 45 min | 99 | — | 84.9 | 64 |
| Fructose | [BMIM]Cl | — | 100 | 10 min | — | — | 94.3 | 112 |
| Fructose | L-Proline | H2O | 90 | 50 min | — | — | 73.6 | 66 |
| Fructose | [BMIM]Cl | DMSO/GeCl4 | 25 | 12 h | — | — | 70 | 68 |
| Fructose | [BMIM][Cl | Amberlyst® 15 | 80 | 10 min | 98.6 | 83.3 | 69 | |
| Fructose | [C4C1im][HSO4] | CrCl3·6H2O | 100 | 3 h | — | — | 96 | 70 |
| Fructose | [BMIM]BF4 | DMSO/Amberlyst 15 | 80 | 32 h | — | — | 87 | 113 |
| Fructose | [C4MIM]Cl | HCl | 80 | 8 min | — | — | 51 | 114 |
| Fructose | [BMIM]Cl | DMSO/solid acid catalyst | 110 | 10 min | 98 | 84 | 115 | |
| Glucose | [AMIM]Cl | DMF | 100 | 45 min | — | — | 0.5 | 64 |
| Glucose | [AMIM]Cl | DMF | 100 | 60 min | — | — | 0.8 | 64 |
| Glucose | [AMIM]Cl | DMF | 100 | 90 min | — | — | 0.9 | 64 |
| Glucose | P[BVIM]Cl | CrCl2 | 120 | 3 h | — | — | 65.8 | 116 |
| Glucose | [BMIM][BF4]/[BMIM][Cl] | Amberlyst 15 | 25 | 2 h | — | — | 32 | 109 |
| Glucose | [BMIM]Cl | CrCl3·6H2O/boric acid | 120 | 30 min | — | — | 78.8 | 117 |
| Glucose | [BMIM]Cl | DMSO/solid acid catalyst | 160 | 50 min | 99 | 68 | 115 | |
| Glucose | [BMIM]Cl | — | 80 | 3 h | — | — | 0.065 | 118 |
| Glucose | [BMIM]Cl | — | 150 | 3 h | — | — | 3 | 118 |
| Glucose | [BMIM]Cl | — | 180 | 3 h | — | — | 2.4 | 118 |
| Glucose | [BMIM]Cl | IrCl3 | 100 | 3 h | — | — | 1.5 | 118 |
| Glucose | [BMIM]Cl | IrCl3 | 120 | 3 h | 49.5 | — | 4.5 | 118 |
| Glucose | [BMIM]Cl | IrCl3 | 140 | 3 h | 69.5 | — | 7.5 | 118 |
| Glucose | [BMIM]Cl | CrCl3 | 100 | 3 h | 78.5 | — | 35.1 | 118 |
| Glucose | [BMIM]Cl | Cr(NO3)3 | 100 | 3 h | 81.9 | — | 37.2 | 118 |
| Glucose | [BMIM]Cl | Zr(NO3)4 | 100 | 3 h | 29.5 | — | 16.7 | 118 |
| Glucose | [BMIM]Cl | Ce(NH4)2(NO3)6 | 100 | 3 h | 4.7 | — | 1.8 | 118 |
| Glucose | [BMIM]Cl | IrCl3 | 120 | 3 h | 90.3 | — | 1.4 | 118 |
| Glucose | [BMIM]Cl | TiO2 + IrCl3 | 150 | 3 h | 59.3 | — | 4.7 | 118 |
| Sucrose | [TetraEG(mim)2][OMs]2− | — | 120 | 150 min | — | — | 67.2 | 82 |
| Sucrose | [C10(Epy)2]2Br− | CrCl2 | 110 | 60 min | 78.5 | 83.9 | 65.8 | 83 |
| Sucrose | [BMIM]Cl | IrCl3 | 80 | 3 h | — | 33.4 | 118 | |
| Sucrose | [BMIM]Cl | IrCl3 | 100 | 3 h | — | 34.2 | 118 | |
| Sucrose | [BMIM]Cl | IrCl3 | 100 | 3 h | — | 37.4 | 118 | |
| Sucrose | [BMIM]Cl | AuCl3·HCl | 100 | 3 h | — | 35.9 | 118 | |
| Sucrose | [BMIM]Cl | 100 | 3 h | — | 2.8 | 118 | ||
| Sucrose | [OMIM][Cl] | CrCl2/HCl | 120 | 30 min | — | 82 ± 3.7 | 119 | |
| Sucrose | [OMIM][Cl] | ZnCl2/HCl | 120 | 30 min | — | 58 ± 2.7 | 119 | |
| Sucrose | [AMIM]Cl | [NMP][HSO4] | 120 | 60 min | 97.2 | — | 82.2 | 119 |
| Sucrose | [AMIM]Cl | [NMP][HSO4] | 120 | 60 min | 97.9 | — | 87 | 111 |
| Sucrose | [AMIM]Cl | [C3SO3HMIM][HSO4] | 120 | 60 min | 98.2 | — | 71.5 | 111 |
| Sucrose | [AMIM]Cl | [C3SO3HMIM][HSO4] | 120 | 60 min | 98.7 | — | 83.1 | 120 |
| Sucrose | [BMIM]Cl | [NMP][HSO4] | 120 | 60 min | 96.6 | — | 73.1 | 120 |
| Sucrose | [BMIM]Cl | [NMP][HSO4] | 120 | 60 min | 96.3 | — | 80.7 | 120 |
| Sucrose | [BMIM]Cl | [C3SO3HMIM][HSO4] | 120 | 60 min | 96 | — | 66 | 120 |
| Sucrose | [BMIM]Cl | [C3SO3HMIM][HSO4] | 120 | 60 min | 97.7 | — | 78.2 | 120 |
| Sucrose | [BMIM]Br | [NMP][HSO4] | 120 | 60 min | 65.3 | — | 77.3 | 120 |
| Sucrose | [BMIM]Br | [C3SO3HMIM][HSO4] | 120 | 60 min | 98.5 | — | 76.2 | 120 |
| Sucrose | [EMIM]Br | [NMP][HSO4] | 120 | 60 min | 95.5 | — | 72.4 | 120 |
| Sucrose | [EMIM]Br | [C3SO3HMIM][HSO4] | 120 | 60 min | 98.1 | — | 69.9 | 120 |
| Sucrose | [AMIM]Cl | H2SO4 | 120 | 60 min | — | — | 50.2 mol% | 120 |
| Sucrose | [AMIM]Cl | H3PW12O40 | 120 | 60 min | — | — | 53.1 mol% | 120 |
| Sucrose | [AMIM]Cl | H3PMoO40 | 120 | 60 min | — | — | 40 mol% | 120 |
| Sucrose | [AMIM]Cl | [MIM][HSO4] | 120 | 60 min | — | — | 36.6 mol% | 120 |
| Sucrose | [AMIM]Cl | Boric acid | 120 | 60 min | — | — | 44.3 mol% | 120 |
| Sucrose | [TetraEG(mim)2][OMs]2− | 120 | 150 min | — | — | 67.2 | 82 | |
| Sucrose | [AMIM]Cl | DMF | 100 | 45 min | — | — | 36.5 | 64 |
| Sucrose | [AMIM]Cl | DMF | 100 | 60 min | — | — | 40.3 | 63 |
| Sucrose | [AMIM]Cl | DMF | 100 | 90 min | — | — | 33.1 | 63 |
Dehydration of fructose to 5 HMF
The first ionic liquid (pyridinium chloride) was used by Fayet and Gelas in fructose dehydration,57 the strong growth of interest towards the use of ionic liquids in biomass conversion started from Moreau's work with imidazolium salts. Matras and Moreau et al.32 carried out fructose dehydration in 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4) with and without DMSO as co-solvent and Amberlyst 15 as catalyst. The yield of HMF 87% was achieved with DMSO at 80 °C in 32 h and 52% was obtained without DMSO in 3 h. The later year Moreau et al.35 reported dehydration of fructose and sucrose in other ionic liquid, a Brønsted acid type of IL, methylimidazolium chloride [HMIM]Cl. More than 90% of the HMF yield was achieved from fructose without additional catalyst. Since then, a variety of ionic liquids have been used in the dehydration of fructose, glucose and polysaccharides.58–60 Recent review papers discussed about one-pot catalytic conversion of carbohydrates into furfural and 5-hydroxymethylfurfural.61 Some more examples are given here from 2010 onwards. Hu et al., reported DMSO as the solvent and an acidic ionic liquid 1-carboxypropyl-3-methyl imidazolium chloride ([CMIm]Cl) as the catalyst. The yield of HMF 95.7% was obtained from fructose when reaction was carried out 120 °C after 120 min. The combination of ZrOCl2 and [CMIm]Cl resulted in a 50.6% yield of HMF from glucose.62Tong et al., reported the dehydration of D-fructose to HMF using catalytic amounts of various Brønsted-acidic ILs such as 1-methylimidazolium hydrogen sulfate ([MIM]+[HSO4]−), N-methyl-2-pyrrolidonium hydrogen sulfate ([NMP]+[HSO4]−), 1-methylimidazolium methyl sulfonate ([MIM]+[CH3SO3]−), and N-methyl-2-pyrrolidonium methyl sulfonate ([NMP]+[CH3SO3]−). The ILs [NMP]+[CH3SO3]− and [NMP]+[HSO4]− have shown the best catalytic activities in the dehydration of D-fructose. The yield of HMF 72.3% with 87.2% selectivity were obtained for 2 h at 90 °C in the presence of 7.5 mol% [NMP]+[CH3SO3]−. They proposed the mechanism for D-fructose dehydration reaction in ionic liquids (Fig. 6).63 In this reaction [NMP]+[HSO4]− and [NMP]+[CH3SO3]− have higher acidity and exhibit better activity compared to [MIM]+-based acidic ILs. The authors say that acidity of [MIM]+[HSO4]− and [MIM]+[CH3SO3]− are very weak; accordingly, their catalytic performances are rather poor in the reaction. [NMP]+[CH3SO3]− was employed as a catalyst, the IL may have interacted with D-fructose or the intermediate through hydrogen bonding and nucleophilic effect, owing to the existence of a carbonyl group and protonated nitrous cation, which led to the production of HMF with high selectivity. Shi et al., developed a catalytic system that made of 1-allyl-3-methylimidazolium chloride ([AMIM]Cl) and N,N-dimethylformamide (DMF) without any additional metal salts or acids, which leads to reasonable HMF yields (84.9%).64 The authors described the mechanism in Fig. 7, chloride ion played an important role in the conversion process. Firstly, a molecule of water removed when compound IM1 formed via the attachment of Cl− to the two OH groups on C1 and C2. Then an enediol intermediate (IM2) is formed and rapidly isomerizes into its isomers aldehyde (IM3). Next, with the formation of another molecule of water which released from IM3, the product IM4 is formed. Then following the similar route above, the third water molecule is removed. Finally, both IL and H2O are dissociated from IM5 to give the target product. It is suggested that in salt solutions with small, strong polarizing cations and large polarizable anions, intensive interactions with OH occur.65 In comparison with [BMIM]Cl, the cation [AMIM]+ is conducive to the attack on OH groups due to the smaller ion size and a double bond in the cation of [AMIM]Cl. Moreover, the less electronic chemical structure caused by allyl group also enhances the interaction between cations in [AMIM]Cl and hydroxyl.
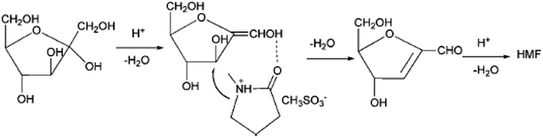 | ||
| Fig. 6 The mechanism for D-fructose dehydration reaction in ionic liquids.63 | ||
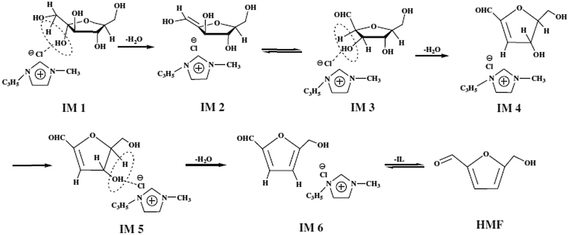 | ||
| Fig. 7 Possible mechanism for the dehydration of fructose. –C3H5 = allyl.64 | ||
Li et al., prepared L-proline derived ionic liquids (ILs) and used as both solvent and catalyst for transformation of fructose and sucrose to 5-hydroxymethylfurfural (HMF) in the presence of water. They employed response surface methodology (RSM) to optimize fructose dehydration process, and a maximum HMF yield of 73.6% could be obtained at 90 °C after 50 min. The recycling of the IL exhibited an almost constant activity during five successive trials, and also they proposed a possible reaction mechanism for the dehydration of fructose to HMF (Fig. 8).66 The mechanism describes that fructose dehydration might be ascribed to the presence of proton and –COOH that can promote the interaction of fructose and the IL via O–H–O hydrogen bonding in the rate-determining step. In the whole catalytic process, three water molecules are successively lost with the assistance of H+ to give the final product HMF.67 The other researcher reported GeCl4 in DMSO and [Bmim]Cl catalytic system gave 70% yield of HMF at 25 °C. The author claims that the structure of ionic liquids and the ratio of ionic liquids to DMSO had a remarkable effect on this new catalytic system. This catalytic system explains that there was a synergistic effect between the cation and anion of [Bmim]Cl on the dehydration of fructose to HMF by designed experiments.68 Qi et al., reported a fructose conversion of 98.6% with a 5-HMF yield of 83.3% in 10 min at 80 °C in ionic liquid 1-butyl-3-methyl imidazolium chloride ([BMIM][Cl]) by using a sulfonic ion exchange resin as catalyst.69 In this catalytic system, no large decrease in 5-HMF selectivity occurred for initial fructose concentrations of up to 20 wt%. Water content of up to 5% in the [BMIM][Cl] had no effect on the fructose conversion rate and 5-HMF yield, but water content higher than 5 wt% led to lower conversions and yields. The ionic liquid and sulfonic ion-exchange resin could be recycled and exhibited constant activity for 7 successive trials. The proposed process of using an ionic liquid with ion-exchange resin catalyst greatly reduces the reaction time required over previous works for converting fructose to 5-HMF. The room-temperature ionic liquid, [C4C1im][HSO4], provides a multi-faceted medium in which to convert fructose to the versatile chemical building block, 5-hydroxymethylfurfural (HMF). Eminov et al., have been investigated a range of metal salts such as CrCl3·6H2O, CrCl3 (anhydrous), MoCl3, Y(OTf)3, RuCl3, NiCl2 and etc., in order to establish some of the properties required for the optimization of this process. This has led to almost quantitative conversion of fructose to 5-HMF in a system that is both selective for the desired product, less energy intensive, and more environmentally benign than the commercial process. Among above all the metal salts, CrCl3·6H2O/IL system showed highest conversion to HMF (80%) after 24 h at 80 °C.70 Zhang et al., describes a dehydration of fructose into 5-hydroxymethylfurfural (HMF) promoted by ionic liquids [Bmim]Cl and [HNMP][CH3SO3] in ethanol solvent. Two intermediates captured in time-dependent HPLC determinations are identified and structurally characterized by in situ 1H NMR and online ESI(+)-MS/MS. Studies on the influence of the ionic liquids (ILs) on the formation and transformation of the intermediates indicate that [HNMP][CH3SO3] promotes the formation of the intermediates, while [Bmim]Cl promotes their transformation. The contribution of the component ions of the ILs to the dehydration originates from their activation toward the leaving of OH on fructose or the intermediates via the formation of multiple hydrogen bonds.71 Liu et al., was used triisobutyl(methyl)phosphonium tosylate (IL) as solvent for conversion of fructose into HMF without any catalyst addition under moderate reaction conditions (80–110 °C). This ionic liquid provides high solubility for sugar and hydroxymethylfurfural (HMF), does not produce heavy or insoluble by-products. This ionic liquid is also tested for conversion of glucose into HMF in presence of CrCl2 catalyst. Formation of some unknown compounds during the sugar conversion is reported first time in the field. In this work, a number of ionic liquid and catalyst combinations are screened using a combinatorial experimental technique with fructose, high fructose corn syrup, and glucose as a feed. Reaction kinetics of sugars in the new ionic liquid is tested in a batch reactor under various reaction conditions, in comparison to [EMIM]Cl – an ionic liquid extensively studied in the previous work. It is found that the catalytic activity of an ionic liquid is determined by choice of both cation and anion, and can also be affected by its production source significantly.72
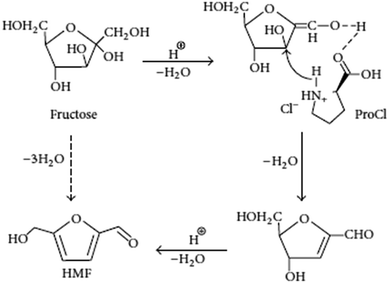 | ||
| Fig. 8 Proposed mechanism for catalytic dehydration of fructose to HMF with ProCl.66 | ||
Zhang and co-workers reported microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid. The HMF and furfural yield of 45–52% and 23–31%, respectively, within 3 min was obtained under the experimental conditions.73 The same group was explored same kind of reaction in different ionic liquids. 1-Butyl-3-methylimidazolium chloride ([Bmim]Cl) catalyzed by ScCl3 under microwave irradiation. Under the optimal reaction conditions, HMF was obtained in a high yield of 73.4% in 2 min with the microwave power at 400 W. Compared with the conventional oil-bath heating manner, the use of microwave irradiation not only reduced reaction times from hours to minutes, but also improved HMF yield.74,75
Some research groups have synthesized dicationic ionic liquids and utilized them in biomass conversion.76–80 The understanding of dicationic ILs, which consists of one dication (two monocations combined into a formation of dications) and two monoanions, is lacking relative to that of monocationic ILs. Actually, dicationic ILs would be rather fascinating research targets. Since the number of possible combinations of cation and anion in dicationic ILs are greater than that in monocationic ILs, a broader variability of the properties of dicationic ILs than monocationic ILs would be possible. Structurally modified dicationic ILs have been successfully applied to the conversion of carbohydrates to HMF.39,81–83 The dehydration of D-fructose has been studied with different kinds of pyridinium, imidazolium based dicationic ionic liquids with symmetry and unsymmetry. Chinnappan et al., reported that 1,10-hexane-1,6-diylbis(3-methylpyridinium) tetrachloronickelate(II) [C6(Mpy)2][NiCl4]2− in DMSO and 1,10-decane-1,10-diylbis(3-ethylpyridinium)dibromide [C10(Epy)2]2Br− without DMSO have high catalytic activity towards fructose dehydration. A fructose conversion of 95.6% and 95.5% HMF yield was achieved by [C6(Mpy)2][NiCl4]2− in 60 min reaction time at 110 °C and 87.1% yield obtained from [C10(Epy)2]2Br− without DMSO at 100 °C in 90 min reaction time.39 In 2012, Arvind et al., used bis(N-methylimidazolium) cations containing short oligo ethylene glycol linkers and mesylate (CH3SO3−) anions based ILs (in catalytic amount) for sugar dehydration reactions. As a result, 92.3% of HMF yield was obtained from fructose in 40 min with one equivalent of [tetraEG(mim)2][OMs]2 at 120 °C. While, 67.2% of HMF was achieved from dehydration of sucrose at 120 °C in 150 min using two equivalents of [tetraEG(mim)2][OMs]2. Among those dicationic RTILs, [tetraEG(mim)2][OMs]2 RTIL was observed to be the most efficient catalyst, demonstrated by its ability to achieve 100% conversion and highest yield of HMF.82 In 2014, Arvind et al., reported the synthesis of unsymmetrical dicationic ILs, imidazolium, pyridinium, and triethyl ammonium as cationic moiety and HSO4, CH3SO3, and Br− as an anionic moiety. The newly synthesized all unsymmetrical ILs were tested for dehydration of fructose into 5-hydroxymethylfurfural (HMF) in mild reaction conditions. Especially ([tetraEG(mim)(triethylamo)]HSO4) unsymmetrical IL was showed higher catalytic activity among all synthesized ILs, it exhibited 100% consumption of fructose and achieved 92.3% HMF yield in 40 min reaction time using 10 wt% of catalyst at 70 °C reaction temperature.81
Glucose and sucrose to HMF
Already numerous catalysts have been studied to the production of HMF from glucose.84–88 Ionic liquids (ILs) are superior in their properties and recognized as excellent solvents and catalysts for conversion of glucose to HMF.89–92 In 2007, Zhao et al.93 reported the conversion of glucose to HMF catalyzed by metal chlorides in ILs as solvent, giving a yield of HMF near 70%. Then Riisager et al.94 was first reported the metal-free conversion of glucose to HMF in ILs, they gave HMF yield of up to 42% by using boric acid as a promoter. Recently, Jiang et al.95 shows a remarkable report that realized the direct conversion of glucose to HMF by solely using acidic ILs, such as 1-butyl-3-methylimidazolium chloride [C4SO3Hmim]Cl, in a single-pot reaction. They found that the yield of total reducing sugar (mainly including glucose and HMF) can be as high as 90%, indicating a promising method for the direct transformation of cellulose into HMF by replacing metal catalysts and inorganic acid catalysts with environmentally benign ILs. This is a ground-breaking technology for such a direct catalytic conversion of glucose to HMF by solely using ILs. Hu et al., reported cellulose-derived carbonaceous catalyst in ionic liquid ([BMIM]Cl) for dehydration of glucose, which resulted in 46.4% HMF yield at 160 °C in 15 min.96 The IL, 1-hydroxyethyl-3-methylimidazolium tetrafluoroborate ([C2OHMIM]BF4), was used a catalyst rather than as a solvent in the conversion of fructose or glucose to 5-HMF. With glucose, the yield of 5-HMF reached as high as 67.3% after 1 h at 180 °C in dimethylsulfoxide (DMSO) as solvent.97 Jadhav et al., was used zeolite (H-ZSM-5) as catalyst and butylmethylimidazolium chloride ([Bmim]Cl) as solvent, leading to a 45% yield of HMF.98 Utami et al. used ytterbium triflate in [Bmim]Cl, this catalytic system showed 52% of HMF yield at 105 °C in 2.7 h.99 Zhang et al.,100 reports the kinetic studies on chromium-catalyzed conversion of glucose into 5-hydroxymethylfurfural in alkylimidazolium chloride ionic liquid. The authors explain the mechanism in three steps: (1) ring opening, (2) hydride transfer between C2 and C1, and (3) ring closure. Glucopyranose (a) is first converted into an acyclic form (b) under the concerted catalysis of Cr(III) alongside Cl (Fig. 9). Cr(III) in chloride-type ILs was reported to be coordinated by six chloride anions by extended X-ray absorption fine structure (EXAFS) measurements. The Cr(III) complexes and Cl acting as electrophile and nucleophile, respectively, simultaneously activate the substrate to promote mutarotation. The following C2 to C1 hydride transfer step involves another glucose molecule. Prior to the H-shift, deprotonation of the O2–H group is required. It has been suggested that the deprotonated glucose intermediate formed in the H shift needs to be stabilized by active-site residues in the enzyme-catalyzed aldose–ketose isomerization. Thus, the other glucose molecule involved here is assumed to help stabilizing the O2/O1-deprotonated glucose intermediate and donate the proton to O-1, which may be the reason for the difference between ILs and the aqueous solution in terms of kinetic order. The resulting C-1 oxyanion is then protonized and transformed into fructofuranose, which is the precursor for the following dehydration.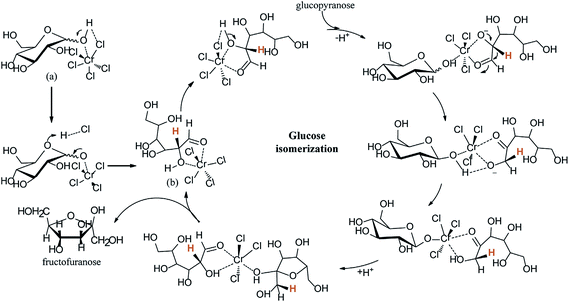 | ||
| Fig. 9 Mechanism for the isomerization of glucose into fructose catalyzed by chromium(III).100 | ||
Li et al., describes the catalytic mechanism of SO3H-functionalized ILs is substantially different from that of the metal chloride for the production of HMF from glucose. They chosen 1-butyl-3-methylimidazolium chloride [C4SO3HmimCl] as a representative of SO3H functionalized IL. This work presents a density functional theory (DFT) study on the catalytic mechanism for conversion of glucose into HMF. It is found that the conversion may proceed via two potential pathways and that throughout most of elementary steps, the cation of the IL plays a substantial role, functioning as a proton shuttle to promote the reaction. The chloride anion interacts with the substrate and the acidic proton in the imidazolium ring via H-bonding, as well as provides a polar environment together with the imidazolium cation to stabilize intermediates and transition states. The general mechanism for the production of HMF from glucose is given in Fig. 10.101
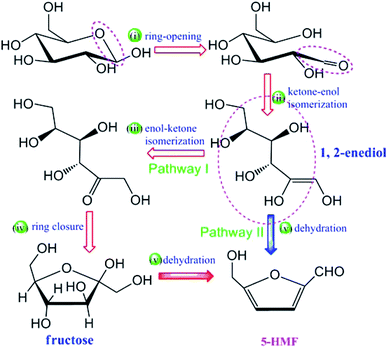 | ||
| Fig. 10 General mechanism for the production of HMF from glucose.101 | ||
Conclusion
Biomass pretreatment has been studied for more than a decade, for the purpose of improving the biodegradability of biomass materials, and currently this important area of research is going through several technological and economic challenges. Considering to the current catalytic system used in biorefining process exhibit some disadvantages such as the use of toxic, flammable, and corrosive solvents, high pressure operations, high water use, pollution, odor and etc. ILs is very good alternative to conventional catalysts and solvents. In carbohydrate transformations, ionic liquids have been showing a phenomenal performance. These solvents can dissolve carbohydrates, even at high concentrations, and can be easily recycled. Moreover, they have been shown not only to act as solvents, but also as reaction promoters for carbohydrate dehydration reactions. They considered as green solvents because of their unique physicochemical properties especially for their low vapor pressure. ILs, as environmentally benign solvents, shows scientific breakthroughs and engineering innovations for the production of biofuels. HMF is an important intermediate chemical, which serves as a platform to synthesize many important industrial chemicals. Most of the ionic liquids along with metal halides were found to be effective for the conversion of carbohydrates into HMF. But recent work has shown that some dicationic ionic liquids are good in this transformation without using additives. This approach leads to reduced reaction costs, and it is important for industry that can use functionalized ILs leading to high yields and selectivity continues. The uses of ionic liquids in biomass treatments are countless. So researcher should design and develop more functionalized ionic liquids containing transition metals. In such cases no need of other additives to use in this transformation. Efficient and environmentally benign transformation of biomass into value-added chemicals and fuels is not only a great importance, but we have a long-time task. The cost of ILs has always been one of the important issues to be considered. Basically some ILs is very expensive compared to traditional solvents at laboratory scale. This is mainly due to the high cost of components as well as the expensive purification process needed for the synthesis of ionic liquids. This is completely depends upon the careful selection of cations and anions. Moreover to reduce the cost of industrial production of HMF and meet the principle of green chemistry, recycling catalysis system is always important. In the synthesis of HMF process, after the reaction, ionic liquids are separated by extraction of products with some solvents like ethyl acetate and water, and after water is removed, the ILs are recycled.102–106Acknowledgements
This study was supported by National Research Foundation of Korea (NRF) – Grants funded by the Ministry of Science, ICT and Future Planning (2014R1A2A2A01004352), Republic of Korea.References
- H. Wang, L. E. Block and R. D. Rogers, Chapter 1: Catalytic Conversion of Biomass in Ionic Liquids, in Catalysis in Ionic Liquids: From Catalyst Synthesis to Application, The Royal Society of Chemistry, 2014, pp. 1–19 Search PubMed.
- R. Luque, L. Herrero-Davila, J. M. Campelo, J. H. Clark, J. M. Hidalgo, D. Luna, J. M. Marinas and A. A. Romero, Energy Environ. Sci., 2008, 1, 542–564 CAS.
- R. Henry, Plant Biotechnol. J., 2010, 8, 288–293 CrossRef CAS PubMed.
- BP Statistical Review of World Energy June 2015, http://www.bp.com/statisticalreview.
- R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Hydroxymethylfurfural, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- C. Baskar, S. Baskar and R. S. Dhillion, Biomass Conversion: The Interface of Biotechnology Chemistry and Materials science, Springer, Heidelberg, New York, Dordrecht, London, 2012 Search PubMed.
- J. Goldemberg, World energy assessment, United Nations Development Programme, New York (USA), 2000 Search PubMed.
- J. J. Bozell, Clean, 2008, 36(8), 641–647 CAS.
- D. Brownlie, J. Soc. Chem. Ind., 1940, 59, 671–675 CrossRef.
- R. Luque, Energy Environ. Sci., 2010, 3, 254–257 CAS.
- S. Dumitriu, Polysaccharides: Structural Diversity and Functional Versatility, CRC Press, 2nd edn, 2012 Search PubMed.
- H. Orgensen, J. B. Kristensen and C. J. Felby, Biofuels, Bioprod. Biorefin., 2007, 1, 119–134 CrossRef.
- E. L. Kunkes, D. A. Simonetti, R. M. West, J. C. Serrano-Ruiz, C. A. Gartner and J. A. Dumesic, Science, 2008, 322, 417–420 CrossRef CAS PubMed.
- Y. Roman-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1936 CrossRef CAS PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- S. Hu, Z. Zhang, J. Song, Y. Zhou and B. Han, Green Chem., 2009, 11, 1746–1749 RSC.
- T. Stahlberg, M. G. Sorensen and A. Riisager, Green Chem., 2010, 12, 321–325 RSC.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- M. Mascal and E. B. Nikitin, Green Chem., 2010, 12, 370–373 RSC.
- T. Stahlberg, W. Fu, J. M. Woodley and A. Riisager, ChemSusChem, 2011, 4, 451–458 CrossRef CAS PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- F. K. Kazi, A. D. Patel, J. C. Serrano-Ruiz, J. A. Dumesic and R. P. Anex, Chem. Eng. J., 2011, 169, 329–338 CrossRef CAS.
- B. Liu and Z. Zhang, ACS Catal., 2016, 6, 326–338 CrossRef CAS.
- Z. Zhang and K. Deng, ACS Catal., 2015, 5, 6529–6544 CrossRef CAS.
- Z. Zhang, J. Zhen, B. Liu, K. Lv and K. Deng, Green Chem., 2015, 17, 1308–1317 RSC.
- S. Yin, J. Sun, B. Liu and Z. Zhang, J. Mater. Chem. A, 2015, 3, 4992–4999 CAS.
- Z. Zhang, Z. Yuan, D. Tang, Y. Ren, K. Lv and B. Liu, ChemSusChem, 2014, 7, 3496–3504 CrossRef CAS PubMed.
- S. P. Teong, G. Yi and Y. Zhang, Green Chem., 2014, 16, 2015–2026 RSC.
- F. S. Asghari and H. Yoshida, Ind. Eng. Chem. Res., 2006, 45, 2163–2173 CrossRef CAS.
- K. Seri, Y. Inoue and H. Ishida, Bull. Chem. Soc. Jpn., 2001, 74, 1145–1150 CrossRef CAS.
- H. Ishida and K. Seri, J. Mol. Catal. A: Chem., 1996, 112, L163–L165 CrossRef CAS.
- C. L. Matras and C. Moreau, Catal. Commun., 2003, 4, 517–520 CrossRef.
- M. A. Schwegler, P. Vinke, M. van der Eijk and H. van Bekkum, Appl. Catal., A, 1992, 80, 41 CrossRef CAS.
- Y. Qu, C. Huang, J. Zhang and B. Chen, Bioresour. Technol., 2012, 106, 170–172 CrossRef CAS PubMed.
- C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165–169 CrossRef CAS.
- P. Carniti, A. Gervasini and M. Marzo, Catal. Commun., 2011, 12, 1122–1126 CrossRef CAS.
- Y. J. Pagan-Torres, T. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef CAS.
- F. S. Asghari and H. Yoshida, Ind. Eng. Chem. Res., 2006, 45, 2163 CrossRef CAS.
- A. Chinnappan, A. H. Jadhav, H. Kim and W. J. Chung, Chem. Eng. J, 2014, 237, 95–100 CrossRef CAS.
- J. N. Chheda, Y. R. Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- M. Bicker, J. Hirth and H. Vogel, Green Chem., 2003, 5, 280–284 RSC.
- B. Liu, Z. Zhang and Z. K. Zhao, Chem. Eng. J, 2013, 215–216, 517–521 CrossRef CAS.
- S. M. dela Rosa, M. Jose, C. Martin and J. L. G. Fierro, Chem. Eng. J, 2012, 181–182, 538–541 CrossRef.
- J. F. Wishart, Energy Environ. Sci., 2009, 2, 956–961 CAS.
- K. N. Marsh, J. A. Boxall and R. Lichtenthaler, Fluid Phase Equilib., 2004, 219, 93–98 CrossRef CAS.
- M. J. Earle and K. R. Seddon, Ionic Liquids: Green Solvents for the Future in Clean Solvents, 2002, ch. 2, pp. 10–25 Search PubMed.
- R. Hayes, G. G. Warr and R. Atkin, Chem. Rev., 2015, 115, 6357–6426 CrossRef CAS PubMed.
- Y. Cao, J. Wu, J. Zhang, H. Li, Y. Zhang and J. He, Chem. Eng. J, 2009, 147, 13–21 CrossRef CAS.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS.
- M. Freemantle, An Introduction to Ionic Liquids, The Royal Society of Chemistry, Cambridge, 2009 Search PubMed.
- J. L. Anderson, R. Ding, A. Ellern and D. W. Armstrong, J. Am. Chem. Soc., 2005, 127, 593–604 CrossRef CAS PubMed.
- J. C. Chang, W. Y. Ho, I. W. Sun, Y. L. Tung, M. C. Tsui, T. Y. Wu and S. S. Liang, Tetrahedron, 2010, 66, 6150–6155 CrossRef CAS.
- T. Payagala, J. Huang, Z. S. Breitbach, P. S. Sharma and D. W. Armstrong, Chem. Mater., 2007, 19, 5848–5850 CrossRef CAS.
- R. Kore and R. Srivastava, J. Mol. Catal. A: Chem., 2011, 345, 10 CrossRef.
- J. C. Chang, W. Y. Ho, I. W. Sun, Y. K. Chou, H. H. Hsieh and T. Y. Wua, Polyhedron, 2011, 30, 497–507 CrossRef CAS.
- C. Fayet and J. Gelas, Carbohydr. Res., 1983, 122, 59–68 CrossRef.
- S. K. Tyrlik, D. Szerszen, M. Olejnik and W. Danikiewicz, J. Mol. Catal. A: Chem., 1996, 106, 223–233 CrossRef CAS.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Energy Fuels, 2010, 24, 737–745 CrossRef CAS.
- C.-Z. Liu, F. Wang, A. R. Stiles and C. Guo, Appl. Energy, 2012, 92, 406–414 CrossRef CAS.
- P. Zhou and Z. Zhang, Catal.: Sci. Technol., 2016, 6, 3694–3712 CAS.
- Z. Hu, B. Liu, Z. Zhang and L. Chen, Ind. Crops Prod., 2013, 50, 264–269 CrossRef CAS.
- X. Tong and Y. Li, ChemSusChem, 2010, 3, 350–355 CrossRef CAS PubMed.
- J. Shi, Y. Yang, N. Wang, Z. Song, H. Gao, Y. Xia, W. Li and H. Wang, Catal. Commun., 2013, 42, 89–92 CrossRef CAS.
- H. Zhang, J. Wu, J. Zhang and J. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- H. Li and S. Yang, Int. J. Chem. Eng., 2014, 978708, DOI:10.1155/2014/978708.
- X. Tong and Y. Li, ChemSusChem, 2010, 3, 350–355 CrossRef CAS PubMed.
- Z. Zhang, B. Liu and Z. K. Zhao, Carbohydr. Polym., 2012, 88, 891–895 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr, Green Chem., 2009, 11, 1327–1331 RSC.
- S. Eminov, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng., 2014, 2, 978–981 CrossRef CAS.
- J. Zhang, X. Yu, F. Zou, Y. Zhong, N. Du and X. Huang, ACS Sustainable Chem. Eng., 2015, 3, 3338–3345 CrossRef CAS.
- W. Liu and J. Holladay, Catal. Today, 2013, 200, 106–116 CrossRef CAS.
- Z. Zhang and Z. K. Zhao, Bioresour. Technol., 2010, 101, 1111–1114 CrossRef CAS PubMed.
- X. Zhou, Z. Zhang, B. Liu, Z. Xu and K. Deng, Carbohydr. Res., 2013, 375, 68–72 CrossRef CAS PubMed.
- Z. Zhang, B. Liu and Z. K. Zhao, Starch/Starke, 2012, 64, 770–775 CrossRef CAS.
- J. Pitawala, A. Matic, A. Martinelli, P. Jacobsson, V. Koch and F. Croce, J. Phys. Chem. B, 2009, 113, 10607 CrossRef CAS PubMed.
- M. Lee, Z. Niu, C. Slebodnick and H. W. Gibson, J. Phys. Chem. B, 2010, 114, 7312 CrossRef CAS PubMed.
- A. Chinnappan and H. Kim, Chem. Eng. J., 2012, 187, 283–288 CrossRef CAS.
- A. Chinnappan, H. Kim, C. Baskar and I. T. Hwang, Int. J. Hydrogen Energy, 2012, 37, 10240–10248 CrossRef CAS.
- A. Chinnappan, H. Kim and I. T. Hwang, Chem. Eng. J., 2012, 191, 451–456 CrossRef CAS.
- A. H. Jadhav, A. Chinnappan, R. H. Patil, S. V. Kostjuk and H. Kim, Chem. Eng. J., 2014, 243, 92–98 CrossRef CAS.
- A. H. Jadhav, H. Kim and I. T. Hwang, Catal. Commun., 2012, 21, 96–103 CrossRef CAS.
- A. Chinnappan, A. H. Jadhav, W.-J. Chung and H. Kim, Ind. Crops Prod., 2015, 76, 12–17 CrossRef CAS.
- G. Yong, Y. G. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS PubMed.
- J. B. Binder and R. T Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC.
- S. Q. Hu, Z. F. Zhang, J. L. Song, Y. X. Zhou and B. X. Han, Green Chem., 2009, 11, 1746–1749 RSC.
- A. M. da Costa Lopes and R. Bogel-Lukasik, ChemSusChem, 2015, 8, 947–965 CrossRef CAS PubMed.
- F. R. Tao, C. Zhuang, Y. Z. Cui and J. Xu, Chin. Chem. Lett., 2014, 25, 757–761 CrossRef CAS.
- Z. H. Zhang, Q. Wang, H. B. Xie, W. J. Liu and Z. B. Zhao, ChemSusChem, 2011, 4, 131–138 CrossRef CAS PubMed.
- Y. S. Qu, C. P. Huang, Y. L. Song, J. Zhang and B. H. Chen, Bioresour. Technol., 2012, 121, 462–466 CrossRef CAS PubMed.
- J. L. Song, H. L. Fan, J. Ma and B. X. Han, Green Chem., 2013, 15, 2619–2635 RSC.
- H. B. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- T. Stahlberg, S. Rodriguez-Rodriguez, P. Fristrup and A. Riisager, Chem–Eur. J., 2011, 17, 1456–1464 CrossRef CAS PubMed.
- F. Jiang, Q. J. Zhu, D. Ma, X. M. Liu and X. W. Han, J. Mol. Catal. A: Chem., 2011, 334, 8–12 CrossRef CAS.
- L. Hu, G. Zhao, X. Tang, Z. Wu, J. Xu, L. Lin and S. Liu, Bioresour. Technol., 2013, 148, 501–507 CrossRef CAS PubMed.
- Y. Qu, C. Huang, Y. Song, J. Zhang and B. Chen, Bioresour. Technol., 2012, 121, 462–466 CrossRef CAS PubMed.
- H. Jadhav, E. Taarning, C. M. Pedersen and M. Bols, Tetrahedron Lett., 2012, 53, 983–985 CrossRef CAS.
- S. P. Utami and N. S. Amin, Ind. Crops Prod., 2013, 41, 64–70 CrossRef CAS.
- J. Zhang, Y. Cao, H. Li and X. Ma, Chem. Eng. J, 2014, 237, 55–61 CrossRef CAS.
- J. Li, J. Li, D. Zhang and C. Liu, J. Phys. Chem. B, 2015, 119, 13398–13406 CrossRef CAS PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–56 CrossRef CAS.
- H. Tadesse and R. Luque, Energy Environ. Sci., 2011, 4, 3913–3929 CAS.
- A. Romero, A. Santos, J. Tojo and A. Rodriguez, J. Hazard. Mater., 2008, 151, 268–273 CrossRef CAS PubMed.
- L. Zhou, R. Liang, Z. Ma, T. Wu and Y. Wu, Bioresour. Technol., 2013, 129, 450–455 CrossRef CAS PubMed.
- C. Li, Z. K. Zhao, H. Cai, A. wang and T. Zhang, Biomass Bioenerg., 2011, 35, 2013–2017 CrossRef CAS.
- J. Shi, Y. Yang, N. Wang, Z. Song, H. Gao, Y. Xia, W. Li and H. Wang, Catal. Commun., 2013, 42, 89–92 CrossRef CAS.
- F. D'Anna, S. Marullo, P. Vitale, C. Rizzo, P. L. Meo and R. Noto, Appl. Catal., A, 2014, 482, 287–293 CrossRef.
- Z. Wei, Y. Li, D. Thushara, Y. Liu and Q. Ren, J. Taiwan Inst. Chem. Eng., 2011, 42, 363–370 CrossRef CAS.
- Y.-S. Qu, Y.-L. Song, C.-P. Huang, J. Zhang and B.-H. Chen, Ind. Eng. Chem. Res., 2012, 51, 13008–13013 CrossRef CAS.
- H. Xie, Z. K. Zhao and Q. Wang, ChemSusChem, 2012, 5, 901–905 CrossRef CAS PubMed.
- S. P. Teong, G. Yi and Y. Zhang, Green Chem., 2014, 16, 2015–2026 RSC.
- C. Li, Z. K. Zhao, A. Wanga, M. Zheng and T. Zhang, Carbohydr. Res., 2010, 345, 1846–1850 CrossRef CAS PubMed.
- F. Guo, Z. Fang and T.-J. Zhou, Bioresour. Technol., 2012, 112, 313–318 CrossRef CAS PubMed.
- D. J. Liu and E. Y.-X. Chen, Biomass Bioenergy, 2013, 48, 181–190 CrossRef CAS.
- L. Hua, Y. Sun, L. Lin and S. Liu, J. Taiwan Inst. Chem. Eng., 2012, 43, 718–723 CrossRef.
- Z. Wei, Y. Li, D. Thushara, Y. Liu and Q. Ren, J. Taiwan Inst. Chem. Eng., 2011, 42, 363–370 CrossRef CAS.
- J.-A. Chun, J.-W. Lee, Y.-B. Yi, S.-S. Hong and C.-H. Chung, Korean J. Chem. Eng., 2010, 27, 930–935 CrossRef CAS.
- J. Shi, W. Liu, N. Wang, Y. Yang and H. Wang, Catal. Lett., 2014, 144, 252–260 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |