Fabrication of TiO2 nanosheets via Ti3+ doping and Ag3PO4 QD sensitization for highly efficient visible-light photocatalysis
Lu Maa,
Hong Hanb,
Lun Pan*a,
Muhammad Tahirac,
Li Wanga,
Xiangwen Zhanga and
Ji-Jun Zoua
aKey Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Collaborative Innovative Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China. E-mail: panlun76@tju.edu.cn
bShandong Engineering Research Center of Chemical Intermediate, Department of Chemistry and Chemical Engineering, Jining University, Qufu 273155, China
cDepartment of Physics, The University of Lahore, 53700, Pakistan
First published on 27th June 2016
Abstract
Visible-light photocatalysis has attracted much attention in environmental remediation and sustainable energy utilization, however, it is still a great challenge to develop highly efficient and stable visible-light photocatalyst. Herein, we developed Ag3PO4 quantum dots (QDs) sensitized and Ti3+-doped TiO2 nanosheets (NS) via a solvothermal/in situ precipitation method. The TiO2/Ag3PO4 ratio in the composite was tuned from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to optimize the dispersion and size of Ag3PO4 QDs, and the best dispersed Ag3PO4 QDs with the smallest size (ca. 2 nm) was obtained for TA1
4 to optimize the dispersion and size of Ag3PO4 QDs, and the best dispersed Ag3PO4 QDs with the smallest size (ca. 2 nm) was obtained for TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The characterizations confirm that abundant Ti3+ defects are introduced into TiO2, and the interaction between Ag3PO4 QDs and TiO2 NS is in the form of Ag–O–Ti bonds, which benefit the visible-light absorption and accelerates the charge separation. Moreover, the well-matched band structures drive the electrons to Ag3PO4 and holes to TiO2 {001} faces, respectively. Therefore, TA1
3. The characterizations confirm that abundant Ti3+ defects are introduced into TiO2, and the interaction between Ag3PO4 QDs and TiO2 NS is in the form of Ag–O–Ti bonds, which benefit the visible-light absorption and accelerates the charge separation. Moreover, the well-matched band structures drive the electrons to Ag3PO4 and holes to TiO2 {001} faces, respectively. Therefore, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 shows a 1.7-fold, 1.4-fold and 5-fold higher activity than bulk Ag3PO4 in MO, phenol photodegradation, and PEC water splitting, respectively. In addition, the sample shows relatively high photostability. Thus, we believe that the rational design of heterostructures based on the matched band and abundant defects can fabricate the highly reactive photocatalysts.
3 shows a 1.7-fold, 1.4-fold and 5-fold higher activity than bulk Ag3PO4 in MO, phenol photodegradation, and PEC water splitting, respectively. In addition, the sample shows relatively high photostability. Thus, we believe that the rational design of heterostructures based on the matched band and abundant defects can fabricate the highly reactive photocatalysts.
1. Introduction
Photocatalysis has attracted considerable attention as it provides a new and benign approach to meet the challenges from the environment, energy and sustainability.1–3 In order to effectively utilize the visible light, a range of photocatalysts have been designed and fabricated, such as Ag3PO4, CdS, WO3, C3N4, doped TiO2 or ZnO, and so on.4–12 Among them, Ag3PO4 has received much attention because of its excellent visible-light response, due to the suitable bandgap (2.45 eV) and very high quantum yield for photooxidation (such as O2 revolution).4,13–16 However, there are several drawbacks that hinder the practical application of Ag3PO4 in photocatalysis: first, the total quantum efficiency of Ag3PO4 is relatively low due to the inherent fast charge recombination. Second, the particle size of Ag3PO4 (0.5 to 2 μm) is too large to achieve high photocatalytic performance.17–19 Third, AgNO3 is a common precursor for the fabrication of Ag3PO4, but it is very expensive, prohibiting the large-scale production of Ag3PO4. To address these issues, many strategies have been developed to rationally design the stable and efficient Ag3PO4-based composites using other band-matched materials, such as BiPO4,20 Fe3O4,21 AgBr,22 ZnO,23 SnO2,24 CdS,16 g-C3N4,7 etc. Specially, the quantum dot (QD) of Ag3PO4 serving for the sensitization to the composites can realize the unilateral charge separation and spatial isolation.Making composite with Ag3PO4, TiO2 nanosheets (TiO2 NS, with {001} facet mainly exposed) is one of the most potential candidates.25 Yang et al. made breakthrough in synthesizing TiO2 NS with high percentage of {001} facets.26 After that, TiO2 NS have been studied extensively in photocatalysis, such as photoelectrochemical (PEC) water splitting for hydrogen production, environmental remediation, CO2 photoreduction and solar cells,8,27–32 attributing to its high photoactivity, nontoxicity, low cost and chemical inertness.33 The in situ observation of fluorogenic reaction confirmed that,34 on a TiO2 NS, the photoinduced electrons preferentially transfer to {101} facets with the holes to {001} facets. Since the exposed surface of {001} faces is more than 80% on TiO2 NS, it can provide abundant hole-trapping sites for charge-pairs isolation. Importantly, the band structure of Ag3PO4 is well matched with TiO2, and the photoinduced holes prefer to transfer from Ag3PO4 to TiO2, with electrons reversely to Ag3PO4.19,35 Therefore, Ag3PO4 QD/TiO2 NS composite will realize the spatial isolation of photoinduced charges.
But TiO2, with the wide band gap of 3.2 eV, cannot be exited by visible light. The Ti3+ doping is an important approach to extend the visible-light absorption,36–39 because of the introduced localized Ti3+ states with energies 0.15–1.18 eV below the conduction band minimum of TiO2. And the relevant experiments confirmed the photoactivity improvement of Ti3+-doped TiO2 under visible light.40
In this work, we fabricate highly photoactive TiO2 NS via Ti3+ doping and Ag3PO4 QDs sensitization, by a solvothermal/in situ precipitation method. The TiO2/Ag3PO4 ratio was tuned to optimize the QDs' size and dispersion, and the interaction between TiO2 and Ag3PO4 was also investigated. The sensitization of Ag3PO4 QDs and introduction of Ti3+ on TiO2 NS result in low charge recombination possibility and unilateral charge transfer, giving rise to the photoactivity of degradation and photoelectrochemical (PEC) water splitting.
2. Experimental
2.1. Materials
CH3COOAg was obtained from Tianjin Kermei Chemical Reagent Co., Ltd. Tetrabutyltitanate (TBT), anhydrous ethanol, NaOH, hydrogen fluoride (HF, 40 wt%), methyl orange (MO), Na2SO4 and Na2HPO4·12H2O were purchased from Tianjin Guangfu Fine Chemical Research Institute. Phenol was obtained from J&K Chemical. Deionized water (>16.0 MΩ cm) was prepared in the lab and used in all experiments. All the reagents were used as received.2.2. Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by modulating the added amount of CH3COOAg, and the resulted samples were named as TA3
4 by modulating the added amount of CH3COOAg, and the resulted samples were named as TA3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, TA1
1, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, TA1
1, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, TA1
3, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, respectively. Pure Ag3PO4 was also prepared using the above mentioned method but without the addition of TiO2 NS.
4, respectively. Pure Ag3PO4 was also prepared using the above mentioned method but without the addition of TiO2 NS.2.3. Characterizations
XRD characterization was conducted using a D/MAX-2500 X-ray diffractometer equipped with Cu Kα radiation. SEM images were observed using a field-emission scanning electron microscope (Hitachi S-4800). High-resolution TEM observations were carried out with a Tecnai G2 F-20 transmission electron microscope. Surface composition and chemical states were analyzed with a PHI-5000 X-ray photoelectron spectroscope (XPS) equipped with Al Kα radiation, and the binding energy was calibrated by the C1s peak (284.6 eV) of the contamination carbon. UV-vis diffuse reflectance spectra (UV-vis DRS) were recorded with a Hitachi U-3010 spectrometer equipped with a 60 mm diameter integrating sphere using BaSO4 as the reflectance sample. Steady-state photoluminescence (PL) spectra were measured by a Horiba JobinYvon Fluorolog3-21.2.4. Photocatalytic reaction
The photocatalytic activities of samples were evaluated by degradation of MO and phenol in a cylindrical quartz vessel under visible light irradiation. 50 mg photocatalyst was dispersed in 100 mL MO (0.06 mM) or phenol (0.2 mM) solution with magnetic stirring. The reactor was kept in dark for 20 min after the adsorption–desorption equilibrium. The reactor was then vertically irradiated by a 300 W high-pressure xenon lamp (CEL-HXUV300, Beijing Aulight Co., Ltd.) with an optical filter (λ ≥ 400 nm). The irradiation area of the light source was ca. 20 cm2. The temperature of the reaction was controlled at 25 ± 1 °C. Samples were withdrawn, centrifuged and analyzed using UV-vis spectrometer (U-3010, Hitachi Ltd.), by monitoring the characteristic absorption wavelength of 463 nm and 270 nm for MO and phenol, respectively.2.5. Photoelectrochemical (PEC)/electrochemical tests
PEC/electrochemical properties were measured using an Ivium Vertex electrochemical workstation in a three-electrode cell with a Pt wire as the counter electrode and an Ag/AgCl reference electrode. Na2SO4 (0.2 M) aqueous solution was used as electrolyte solution. The working electrode was prepared by dip-coating sample slurry on an F-doped tin oxide (FTO) glass electrode (1 cm × 1 cm). Electrochemical impedance spectroscopy (EIS) measurements were carried out with a sinusoidal ac perturbation of 10 mV applied over the frequency range of 0.01–105 Hz.3. Result and discussion
3.1. Crystal structure
The XRD patterns of prepared samples are shown in Fig. 1. The TiO2 NS is in the phase of anatase, with the main diffraction peak at 2θ = 25.3° ([101] plane). After the decoration of Ag3PO4, its diffraction peaks appear with the main peak at 2θ = 33.3° ([210] plane), and the peak intensity gradually increases with the decrease of TiO2/Ag3PO4 mass ratio. Finally, the pure Ag3PO4 is produced when no TiO2 NS are added into the synthetic solution. For Ag3PO4 and Ag3PO4/TiO2 composites, no diffraction peaks of metallic Ag or other impurities have been detected.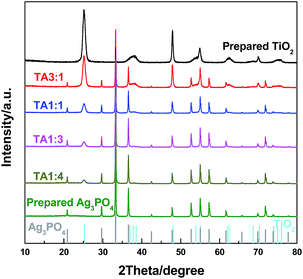 | ||
| Fig. 1 XRD patterns of TiO2, Ag3PO4 and Ag3PO4/TiO2 composites, the standard XRD patterns of Ag3PO4 and TiO2 anatase are based on JCPDS card no. 06-0505 and no. 21-1272, respectively. | ||
3.2. Sample morphology
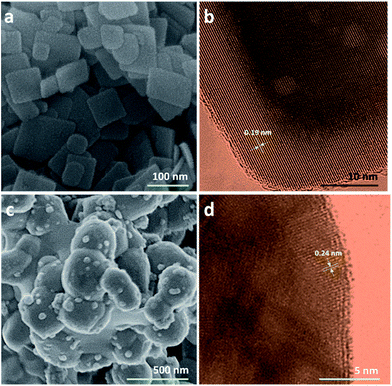
As shown in Fig. 2a, SEM images show that pure TiO2 are square nanosheets with the average length of ca. 70 nm and the thickness of ca. 8 nm, which are the same results with the previous work.27 For pure Ag3PO4 (Fig. 2c), the diameter of irregular spherical particles is about 30–400 nm, and the serious aggregation is observed. From TEM images (Fig. 2b and d), pure TiO2 and Ag3PO4 both show high degree of crystallinity. The lattice fringe spacing of 0.19 nm (Fig. 2b) is attributed to anatase (200) and (020) atomic planes, indicating TiO2 NS are mainly exposed with {001} facets.41 In Fig. 2d, the lattice fringe of 0.24 nm is referred to Ag3PO4 (211) planes.15The aggregation of Ag3PO4 is harmful for photoactivity, while the well dispersed nanoparticles are very promising.42 Hereby, TiO2 NS serve as the support for the well dispersed growth of Ag3PO4 nanoparticles. As shown in Fig. 3, the decorated Ag3PO4 are nanoparticles and their aggregations have been inhibited effectively. There are no obvious size changes for TiO2 NS, while the average size of Ag3PO4 nanoparticles is dependent on its deposited amount. Under low amount loading (TA3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Fig. 3a–c), Ag3PO4 nanoparticles loosely disperse on TiO2 NS, and they have the wide size range from 2–14 nm with the average size of 6 nm. With the increase of Ag3PO4 loading amount (TA1
1, Fig. 3a–c), Ag3PO4 nanoparticles loosely disperse on TiO2 NS, and they have the wide size range from 2–14 nm with the average size of 6 nm. With the increase of Ag3PO4 loading amount (TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Fig. 3d–f; and TA1
1, Fig. 3d–f; and TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, Fig. 3g–i), the average particle size of Ag3PO4 is reduced to 4.5 nm and 2 nm, respectively, and their size distribution becomes narrower, especially for TA1
3, Fig. 3g–i), the average particle size of Ag3PO4 is reduced to 4.5 nm and 2 nm, respectively, and their size distribution becomes narrower, especially for TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. However, further increase of Ag3PO4 loading amount (TA1
3. However, further increase of Ag3PO4 loading amount (TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) causes the aggregation of Ag3PO4 nanoparticles with the average size of 4.2 nm (Fig. 3j–l). Accordingly, using TiO2 NS as support, Ag3PO4 QDs have been successfully fabricated. For all samples, Ag3PO4 QDs show high degree of crystallinity with the lattice fringe spacing of 0.24 nm ((211) planes).
4) causes the aggregation of Ag3PO4 nanoparticles with the average size of 4.2 nm (Fig. 3j–l). Accordingly, using TiO2 NS as support, Ag3PO4 QDs have been successfully fabricated. For all samples, Ag3PO4 QDs show high degree of crystallinity with the lattice fringe spacing of 0.24 nm ((211) planes).
 | ||
Fig. 3 TEM images and size distribution of Ag3PO4 for TA3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (a–c), TA1 1 (a–c), TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (d–f), TA1 1 (d–f), TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (g–i) and TA1 3 (g–i) and TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (j–l). 4 (j–l). | ||
3.3. Surface composition
As shown in Fig. 4, XPS characterizations of TiO2, Ag3PO4 and TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were conducted to investigate the chemical composition and status of the materials. For pure Ag3d spectra of Ag3PO4 (Fig. 4a), the peaks at ca. 373.8 eV and 367.7 eV are assigned to Ag3d3/2 and Ag3d5/2 of Ag+, respectively.19,43 After the loading of Ag3PO4 QDs on TiO2 NS (TA1
3 were conducted to investigate the chemical composition and status of the materials. For pure Ag3d spectra of Ag3PO4 (Fig. 4a), the peaks at ca. 373.8 eV and 367.7 eV are assigned to Ag3d3/2 and Ag3d5/2 of Ag+, respectively.19,43 After the loading of Ag3PO4 QDs on TiO2 NS (TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, Fig. 4e), the locations of Ag3d3/2 and Ag3d5/2 attributing to Ag+ are almost the same as that of pure Ag3PO4, while the new peaks at 365.9 eV and 371.8 eV are owing to the formed Ag–O–Ti bonds.43 The low-energy shift of Ag+ binding energy in the form of Ag–O–Ti is resulted from the weaker electrical negative of Ti than that of P. The presence of the Ti–O–Ag bonds indicates a strong covalent interaction exists between Ag3PO4 QDs and TiO2 NS, confirming the formation of the heterojunction structure, which is vital for electron–hole separation.44
3, Fig. 4e), the locations of Ag3d3/2 and Ag3d5/2 attributing to Ag+ are almost the same as that of pure Ag3PO4, while the new peaks at 365.9 eV and 371.8 eV are owing to the formed Ag–O–Ti bonds.43 The low-energy shift of Ag+ binding energy in the form of Ag–O–Ti is resulted from the weaker electrical negative of Ti than that of P. The presence of the Ti–O–Ag bonds indicates a strong covalent interaction exists between Ag3PO4 QDs and TiO2 NS, confirming the formation of the heterojunction structure, which is vital for electron–hole separation.44
 | ||
Fig. 4 XPS spectra of Ag3PO4 (a–c), TiO2 NS (d) and TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (e–h): (a and e) Ag3d spectra; (b and f) O1s spectra; (c and g) P2p spectra; (d and h) Ti2p spectra. 3 (e–h): (a and e) Ag3d spectra; (b and f) O1s spectra; (c and g) P2p spectra; (d and h) Ti2p spectra. | ||
Fig. 4b shows the high-resolution O1s spectra of pure Ag3PO4, with a symmetric peak located at ca. 531.3 eV, which is attributed to O–P species.43 However, the decoration of Ag3PO4 QDs on TiO2 NS alters the chemical environments of O species (Fig. 4f). The asymmetric peak is divided into four peaks centered at 532.8 eV, 531.2 eV, 530.2 eV and 529.0 eV, which are related to oxygen species in adsorbed H2O, P–O, Ti–O–Ag and Ti–O moieties, respectively.42,43,45 Similar to the results of Ag3d spectra, the appearance of Ti–O–Ag suggests the interaction between Ag3PO4 QDs and TiO2 NS. P2p spectra of pure Ag3PO4 and TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 are shown in Fig. 4c and g. These two materials both show one peak with the binding energy of ca. 132.5 eV, attributing to P5+ in PO43−.43
3 are shown in Fig. 4c and g. These two materials both show one peak with the binding energy of ca. 132.5 eV, attributing to P5+ in PO43−.43
The oxidation state of Ti element in TiO2 NS is shown in Fig. 4d, two kinds of Ti species are found. The peaks at the binding energy of 458.8 eV (Ti2p3/2) and 464.3 eV (Ti 2p1/2), and their splitting of 5.7 eV are referred to Ti4+ species of bulk TiO2.27,43,45 The other is the defected Ti3+ with the binding energy centered at 457.7 eV and 463.7 eV.27,45 This kind of Ti3+ defects are caused during the hydrothermal treatment in the presence of HF. Previous calculation confirmed that Ti3+ tends to be stabilized at five-coordinated surface sites,46 and actually, all surface Ti atoms on {001} TiO2 NS are five-coordinated.47 As shown in Fig. 4h, Ag3PO4 QDs-TiO2 NS (TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) still maintain the doped Ti3+. But the characteristic peaks of Ti2p1/2 and Ti2p3/2 become broader as compared to pure TiO2 NS, which indicates the existence of new Ti species. There are two new peaks located at 459.8 eV and 466.9 eV, which are owing to the bond of Ti–O–Ag.43 The higher binding energy of Ti in Ti–O–Ag is resulted from the stronger electrical negative of Ag than Ti. From the above XPS results of TA1
3) still maintain the doped Ti3+. But the characteristic peaks of Ti2p1/2 and Ti2p3/2 become broader as compared to pure TiO2 NS, which indicates the existence of new Ti species. There are two new peaks located at 459.8 eV and 466.9 eV, which are owing to the bond of Ti–O–Ag.43 The higher binding energy of Ti in Ti–O–Ag is resulted from the stronger electrical negative of Ag than Ti. From the above XPS results of TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, it can be seen that Ti3+–TiO2 NS and Ag3PO4 QDs are not just physically mixed, however, they have chemical bond of Ti–O–Ag, suggesting that Ag3PO4 QDs have been effectively loaded on Ti3+–TiO2 NS.
3, it can be seen that Ti3+–TiO2 NS and Ag3PO4 QDs are not just physically mixed, however, they have chemical bond of Ti–O–Ag, suggesting that Ag3PO4 QDs have been effectively loaded on Ti3+–TiO2 NS.
3.4. Optical absorption and charge separation
UV-vis DRS spectra of Ag3PO4, TiO2 and Ag3PO4 QDs-TiO2 NS are exhibited in Fig. 5a. It clearly shows that bulk Ag3PO4 can absorb visible light with a wavelength less than 546 nm, corresponding to the band gap of ca. 2.27 eV,19 while TiO2 NS have an absorption edge at ca. 420 nm, with the band gap of ca. 2.95 eV. The narrowed band gap is owing to the Ti3+ doping.1 With the gradually increasing amount of Ag3PO4 QDs in the composites, the absorption edges are red-shifted and the calculated band gap energy (inset in Fig. 5a) is obviously decreased. The introduction of Ti3+ and loading of Ag3PO4 QDs on TiO2 NS make the composite to show optical absorption in the visible-light range, and this will increase the visible-light catalytic activity of TiO2 NS. | ||
| Fig. 5 (a) UV-vis DRS and (b) steady-state PL spectra of Ag3PO4 QDs-TiO2 NS composites. The inset in (a) the corresponding plots of transformed Kubelka–Munk function versus the photo energy. | ||
The efficient separation of photo-induced charges is vital for the high photocatalytic activity.3 The photoluminescence (PL) spectra of Ag3PO4 and Ag3PO4 QDs-TiO2 NS composites are tested (Fig. 5b). The emission peaks at 420 nm are corresponding to the shallow donor level of Ti3+ state to the valence band (2.95 eV). The Ag3PO4 has the strongest emission band between 450 and 750 nm, due to the rapid recombination of excited electrons and holes (2.22 eV). The results are consistent with the UV-vis absorption. Moreover, the decoration of Ag3PO4 QDs on TiO2 NS greatly influences the PL intensity, with obvious decrease of PL intensity for the composites as compared to pure Ag3PO4. Initially, the emission peak intensity gradually declines with the decrease of TiO2/Ag3PO4 ratio. Especially when the ratio of TiO2/Ag3PO4 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the PL emission intensity is the lowest. However, when TiO2/Ag3PO4 ratio is lower than 1
3, the PL emission intensity is the lowest. However, when TiO2/Ag3PO4 ratio is lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, such as TA1
3, such as TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the PL emission intensity increases again, but is still lower than pure Ag3PO4. The PL spectra indicate that the composition of Ag3PO4 QDs and TiO2 NS inhibits the recombination of photoinduced charge pairs, and TA1
4, the PL emission intensity increases again, but is still lower than pure Ag3PO4. The PL spectra indicate that the composition of Ag3PO4 QDs and TiO2 NS inhibits the recombination of photoinduced charge pairs, and TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 possesses the highest charge-separation efficiency.
3 possesses the highest charge-separation efficiency.
3.5. Photocatalytic performance
The model photocatalytic reactions, degradation of organic compounds (methyl orange-MO and phenol), were conducted to evaluate the photoactivity of as-synthesized samples. During the photodegradation, the formation of hydroxyl radical (˙OH) caused by electrons and holes is crucial.33,48–50 Another important model, photoelectrochemical (PEC) water splitting, is an important model to evaluate the activity of photocatalyst.48–51 It is emerging as the promising methods for solar hydrogen and oxygen generation, and has attracted increasing interest. In most case, the performance of PEC cell is largely determined by the properties of the electrode. Hence in this work, the above two typical photocatalytic tests were carried out to evaluate the photoactivity of the materials.The photocatalytic activities of TiO2, Ag3PO4, and Ag3PO4 QDs-TiO2 NS composites were firstly evaluated by the degradation of MO and phenol under visible-light irradiation (Fig. 6a–d). Before irradiation, the samples were stirred in the dark for 20 min to reach adsorption–desorption equilibrium. Under visible-light irradiation, TiO2 NS without Ti3+, treated by 5 h thermal calcination under 500 °C, show very low visible-light activity with only ca. 1.5% MO degraded and almost no degradation of phenol observed in 100 min. But, TiO2 NS with abundant Ti3+ shows obvious visible-light-catalytic activity, with the reaction rate constant (k) of 0.002 min−1 (MO degradation) and 0.004 min−1 (phenol degradation). Therefore, Ti3+ doping plays an important role in visible-light response for TiO2. Meanwhile, Ag3PO4 shows relatively high photoactivity with k of 0.022 min−1 for (MO degradation) and 0.021 min−1 for phenol degradation, respectively.
Ag3PO4 QDs sensitized TiO2 NS exhibit much higher photocatalytic activity than TiO2 NS. With the increase of Ag3PO4 QDs amount, from TA3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to TA1
1 to TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and then to TA1
1 and then to TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the photoactivities are enhanced significantly (Fig. 6a–d). Particularly, TA1
3, the photoactivities are enhanced significantly (Fig. 6a–d). Particularly, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 exhibits the highest activity with k values of 0.037 min−1 for MO degradation and 0.030 min−1 for phenol degradation, which are respectively 1.7-fold and 1.4-fold higher than those of bulk Ag3PO4. However, further increasing the amount of Ag3PO4 QDs causes the decrement of photoactivity, such as TA1
3 exhibits the highest activity with k values of 0.037 min−1 for MO degradation and 0.030 min−1 for phenol degradation, which are respectively 1.7-fold and 1.4-fold higher than those of bulk Ag3PO4. However, further increasing the amount of Ag3PO4 QDs causes the decrement of photoactivity, such as TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
The similar trend is also observed in photoelectrochemical (PEC) water splitting (Fig. 6e), TiO2 NS exhibit the visible-light response but the photocurrent density is ca. 8 μA cm−2 (at 1.0 V vs. RHE), while that for Ag3PO4 is ca. 10 μA cm−2. Interestingly, the composite, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, shows very high PEC performance with the photocurrent density of ca. 52 μA cm−2 (at 1.0 V vs. RHE), which is about 5-fold higher than that of pure Ag3PO4. The above results indicate that the loading amount of Ag3PO4 QDs on TiO2 NS is very important for the high photoactivity.
3, shows very high PEC performance with the photocurrent density of ca. 52 μA cm−2 (at 1.0 V vs. RHE), which is about 5-fold higher than that of pure Ag3PO4. The above results indicate that the loading amount of Ag3PO4 QDs on TiO2 NS is very important for the high photoactivity.
With smaller size and better dispersion of Ag3PO4 QDs, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 provides more active sites for reactant adsorption, mass transfer and further degradation. Hence, the composite structure with appropriate amount and well dispersion of Ag3PO4 QDs is optimal to achieve high photoactivity. Besides of the structure, the charge-separation efficiency also plays the important role in photocatalysis. To improve the effective charge separation, the formation of heterostructure is one of the most potential strategy.33 As shown in Fig. 7, the VB and CB edge potentials of Ag3PO4 are 2.9 eV and 0.45 eV, respectively, while those of TiO2 are 2.7 eV and −0.25 eV (for Ti3+ state), respectively, which are both negative to that of Ag3PO4.43 Therefore, Ti3+–TiO2 and Ag3PO4 can form well matched heterostructure. When the composite is irradiated by visible light, the electrons of Ag3PO4 VB can be excited to CB and creates holes in VB. Then, the holes are rapidly transferred to VB of TiO2 {001} facets, while the electrons are maintained in Ag3PO4 CB. In contrast, the photoinduced electrons of Ti3+–TiO2 will transfer to Ag3PO4 QDs, with the holes in VB. In this case, the charge-pairs separation and spatial location realize on the composite under visible light irradiation. Importantly, the key to obtain high charge-separation efficiency is the well dispersion of Ag3PO4 QDs on TiO2 NS. Therefore, TA1
3 provides more active sites for reactant adsorption, mass transfer and further degradation. Hence, the composite structure with appropriate amount and well dispersion of Ag3PO4 QDs is optimal to achieve high photoactivity. Besides of the structure, the charge-separation efficiency also plays the important role in photocatalysis. To improve the effective charge separation, the formation of heterostructure is one of the most potential strategy.33 As shown in Fig. 7, the VB and CB edge potentials of Ag3PO4 are 2.9 eV and 0.45 eV, respectively, while those of TiO2 are 2.7 eV and −0.25 eV (for Ti3+ state), respectively, which are both negative to that of Ag3PO4.43 Therefore, Ti3+–TiO2 and Ag3PO4 can form well matched heterostructure. When the composite is irradiated by visible light, the electrons of Ag3PO4 VB can be excited to CB and creates holes in VB. Then, the holes are rapidly transferred to VB of TiO2 {001} facets, while the electrons are maintained in Ag3PO4 CB. In contrast, the photoinduced electrons of Ti3+–TiO2 will transfer to Ag3PO4 QDs, with the holes in VB. In this case, the charge-pairs separation and spatial location realize on the composite under visible light irradiation. Importantly, the key to obtain high charge-separation efficiency is the well dispersion of Ag3PO4 QDs on TiO2 NS. Therefore, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 shows the lowest emission intensity in PL spectra (Fig. 5b). In addition, the electrochemical impedance spectroscopy (EIS) technique was also used to elucidate the kinetics and mechanism of photocatalytic performance, and smaller arc radius implies smaller charge transfer resistance. Since the charge transfer resistance (Rct) value is inversely proportional to the electron transfer rate, TA1
3 shows the lowest emission intensity in PL spectra (Fig. 5b). In addition, the electrochemical impedance spectroscopy (EIS) technique was also used to elucidate the kinetics and mechanism of photocatalytic performance, and smaller arc radius implies smaller charge transfer resistance. Since the charge transfer resistance (Rct) value is inversely proportional to the electron transfer rate, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 has the fastest electron transfer rate, which is consistent with PL spectra and the photocatalytic performance.
3 has the fastest electron transfer rate, which is consistent with PL spectra and the photocatalytic performance.
 | ||
| Fig. 7 Schematic diagram of the photocatalytic mechanism of Ag3PO4 QDs-TiO2 NS composite under visible light irradiation. | ||
Finally, the photostability of TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was tested in MO photodegradation (Fig. 8a) and PEC performance (Fig. 6e). After 5 cycles for photodegradation and 10 cycles for PEC water splitting, TA1
3 was tested in MO photodegradation (Fig. 8a) and PEC performance (Fig. 6e). After 5 cycles for photodegradation and 10 cycles for PEC water splitting, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 still retains more than 90% of the initial photoactivity. For the recycled samples, no other phases besides Ag3PO4 and TiO2 anatase are observed from the XRD patterns (Fig. 8b), and the doped Ti3+ are also very stable (Fig. 8c). But the careful characterization of Ag3d XPS shows the appearance of trace Ag0 in the recycled sample (Fig. 8d), which is the reason for the indistinctively decreased stability in photocatalysis.
3 still retains more than 90% of the initial photoactivity. For the recycled samples, no other phases besides Ag3PO4 and TiO2 anatase are observed from the XRD patterns (Fig. 8b), and the doped Ti3+ are also very stable (Fig. 8c). But the careful characterization of Ag3d XPS shows the appearance of trace Ag0 in the recycled sample (Fig. 8d), which is the reason for the indistinctively decreased stability in photocatalysis.
 | ||
Fig. 8 5-Cycle photodegradation of MO catalyzed by TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 under visible light (a), XRD patterns (b), and Ti2p (c) and Ag3d (d) XPS spectra of recycled TA1 3 under visible light (a), XRD patterns (b), and Ti2p (c) and Ag3d (d) XPS spectra of recycled TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. 3. | ||
4. Conclusions
Ag3PO4 QDs sensitized and Ti3+ doped TiO2 NS have been successfully synthesized via a solvothermal/in situ precipitation approach. The TiO2/Ag3PO4 ratio was tuned from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to optimize the composite heterostructure, and the well dispersed Ag3PO4 QDs with small size of ca. 2 nm was obtained for TA1
4 to optimize the composite heterostructure, and the well dispersed Ag3PO4 QDs with small size of ca. 2 nm was obtained for TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. XPS data indicate Ag3PO4 QDs and Ti3+–TiO2 NS are not simply physical mixture but interact with each other in the form of Ag–O–Ti connections, which can accelerate the charge separation and transfer between the well matched band structures of Ag3PO4 QDs and Ti3+–TiO2 NS. Therefore, TA1
3. XPS data indicate Ag3PO4 QDs and Ti3+–TiO2 NS are not simply physical mixture but interact with each other in the form of Ag–O–Ti connections, which can accelerate the charge separation and transfer between the well matched band structures of Ag3PO4 QDs and Ti3+–TiO2 NS. Therefore, TA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 shows 1.7-fold, 1.4-fold and 5-fold higher activity than bulk Ag3PO4 in MO, phenol photodegradation, and PEC water splitting, respectively. In addition, Ag3PO4 QDs-TiO2 NS composite shows excellent photostability. Our work reveals that the rational design of heterostructure based on the matched band structure along with abundant defects can fabricate the efficient materials for photocatalysis.
3 shows 1.7-fold, 1.4-fold and 5-fold higher activity than bulk Ag3PO4 in MO, phenol photodegradation, and PEC water splitting, respectively. In addition, Ag3PO4 QDs-TiO2 NS composite shows excellent photostability. Our work reveals that the rational design of heterostructure based on the matched band structure along with abundant defects can fabricate the efficient materials for photocatalysis.
Acknowledgements
The authors appreciate the supports from the National Natural Science Foundation of China (21506156, U1462119), the Tianjin Municipal Natural Science Foundation (15JCZDJC37300, 16JCQNJC05200), Shandong Provincial Natural Science Foundation (ZR2015PB006, ZR2015BL022), and Science and Technology Key Projects of Shandong Province (2014GGH217001).Notes and references
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- G. Li, Y. Wang and L. Mao, RSC Adv., 2014, 4, 53649–53661 RSC.
- L. Pan, X. Zhang, L. Wang and J.-J. Zou, Mater. Lett., 2015, 160, 576–580 CrossRef CAS.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2011, 41, 782–796 RSC.
- Z.-F. Huang, J. Song, L. Pan, X. Zhang, L. Wang and J.-J. Zou, Adv. Mater., 2015, 27, 5309–5327 CrossRef CAS PubMed.
- D. Venieri, I. Gounaki, V. Binas, A. Zachopoulos, G. Kiriakidis and D. Mantzavinos, Appl. Catal., B, 2015, 178, 54–64 CrossRef CAS.
- J. Yu, J. Fan and K. Lv, Nanoscale, 2010, 2, 2144–2149 RSC.
- J.-K. Yan, K.-Y. Kang and G.-Y. Gan, Mater. Des., 2016, 99, 155–162 CrossRef CAS.
- R. Fagan, D. E. McCormack, S. Hinder and S. C. Pillai, Mater. Des., 2016, 96, 44–53 CrossRef CAS.
- F. Achouri, S. Corbel, L. Balan, K. Mozet, E. Girot, G. Medjahdi, M. B. Said, A. Ghrabi and R. Schneider, Mater. Des., 2016, 101, 309–316 CrossRef CAS.
- L. Pan, T. Muhammad, L. Ma, Z.-F. Huang, S. Wang, L. Wang, J.-J. Zou and X. Zhang, Appl. Catal., B, 2016, 189, 181–191 CrossRef CAS.
- H. Yu, G. Cao, F. Chen, X. Wang, J. Yu and M. Lei, Appl. Catal., B, 2014, 160–161, 658–665 CrossRef CAS.
- H. Yu, L. Xu, P. Wang, X. Wang and J. Yu, Appl. Catal., B, 2014, 144, 75–82 CrossRef CAS.
- P. Dong, Y. Wang, B. Cao, S. Xin, L. Guo, J. Zhang and F. Li, Appl. Catal., B, 2013, 132–133, 45–53 CrossRef CAS.
- L. Qi, J. Yu and M. Jaroniec, Phys. Chem. Chem. Phys., 2011, 13, 8915–8923 RSC.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- Y. Bi, H. Hu, S. Ouyang, G. Lu, J. Cao and J. Ye, Chem. Commun., 2012, 48, 3748–3750 RSC.
- F.-M. Zhao, L. Pan, S. Wang, Q. Deng, J.-J. Zou, L. Wang and X. Zhang, Appl. Surf. Sci., 2014, 317, 833–838 CrossRef CAS.
- H. Lin, H. Ye, B. Xu, J. Cao and S. Chen, Catal. Commun., 2013, 37, 55–59 CrossRef CAS.
- G. Li and L. Mao, RSC Adv., 2012, 2, 5108–5111 RSC.
- Y. Bi, S. Ouyang, J. Cao and J. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071–10075 RSC.
- W. Liu, M. Wang, C. Xu, S. Chen and X. Fu, Mater. Res. Bull., 2013, 48, 106–113 CrossRef CAS.
- L. Zhang, H. Zhang, H. Huang, Y. Liu and Z. Kang, New J. Chem., 2012, 36, 1541–1544 RSC.
- M. Xing, J. Zhang, B. Qiu, B. Tian, M. Anpo and M. Che, Small, 2015, 11, 1920–1929 CrossRef CAS PubMed.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638–641 CrossRef CAS PubMed.
- L. Pan, J.-J. Zou, S. Wang, Z.-F. Huang, A. Yu, L. Wang and X. Zhang, Chem. Commun., 2013, 49, 6593–6595 RSC.
- X. Li, J. Wang, Y. Men and Z. Bian, Appl. Catal., B, 2016, 187, 115–121 CrossRef CAS.
- D. Sun, W. Yang, L. Zhou, W. Sun, Q. Li and J. K. Shang, Appl. Catal., B, 2016, 182, 85–93 CrossRef CAS.
- L. Ren, Y. Li, J. Hou, J. Bai, M. Mao, M. Zeng, X. Zhao and N. Li, Appl. Catal., B, 2016, 181, 625–634 CrossRef CAS.
- L. Liu, Y. Jiang, H. Zhao, J. Chen, J. Cheng, K. Yang and Y. Li, ACS Catal., 2016, 6, 1097–1108 CrossRef CAS.
- G. Liu, H. G. Yang, J. Pan, Y. Q. Yang, G. Q. Lu and H.-M. Cheng, Chem. Rev., 2014, 114, 9559–9612 CrossRef CAS PubMed.
- A. Kubacka, M. Fernández-García and G. Colón, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- T. Tachikawa, S. Yamashita and T. Majima, J. Am. Chem. Soc., 2011, 133, 7197–7204 CrossRef CAS PubMed.
- W. Yao, B. Zhang, C. Huang, C. Ma, X. Song and Q. Xu, J. Mater. Chem., 2012, 22, 4050–4055 RSC.
- F. Zuo, L. Wang, T. Wu, Z. Zhang, D. Borchardt and P. Feng, J. Am. Chem. Soc., 2010, 132, 11856 CrossRef CAS PubMed.
- M. Liu, X. Qiu, M. Miyauchi and K. Hashimoto, Chem. Mater., 2011, 23, 5282–5286 CrossRef CAS.
- M. S. Hamdy, R. Amrollahi and G. Mul, ACS Catal., 2012, 2, 2641–2647 CrossRef CAS.
- M. Xing, W. Fang, M. Nasir, Y. Ma, J. Zhang and M. Anpo, J. Catal., 2013, 297, 236–243 CrossRef CAS.
- B. Qiu, Y. Zhou, Y. Ma, X. Yang, W. Sheng, M. Xing and J. Zhang, Sci. Rep., 2015, 5, 8591 CrossRef CAS PubMed.
- X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2009, 131, 3152–3153 CrossRef CAS PubMed.
- W. Teng, X. Li, Q. Zhao and G. Chen, J. Mater. Chem. A, 2013, 1, 9060–9068 CAS.
- Z. W. Tong, D. Yang, Y. Y. Sun, Y. Tian and Z. Y. Jiang, Phys. Chem. Chem. Phys., 2015, 17, 12199–12206 RSC.
- X. Yang, J. Qin, Y. Jiang, K. Chen, X. Yan, D. Zhang, R. Li and H. Tang, Appl. Catal., B, 2015, 166–167, 231–240 CrossRef CAS.
- L. Pan, S. Wang, J.-J. Zou, Z.-F. Huang, L. Wang and X. Zhang, Chem. Commun., 2014, 50, 988–990 RSC.
- Y. Wang, H. Zhang, Y. Han, P. Liu, X. Yao and H. Zhao, Chem. Commun., 2011, 47, 2829 RSC.
- J. Pan, G. Liu, G. Q. Lu and H.-M. Cheng, Angew. Chem., Int. Ed., 2011, 50, 2133–2137 CrossRef CAS PubMed.
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- L. Pan, J.-J. Zou, X. Zhang and L. Wang, J. Am. Chem. Soc., 2011, 133, 10000–10002 CrossRef CAS PubMed.
- S. Wang, L. Pan, J.-J. Song, W. Mi, J.-J. Zou, L. Wang and X. Zhang, J. Am. Chem. Soc., 2015, 137, 2975–2983 CrossRef CAS PubMed.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
| This journal is © The Royal Society of Chemistry 2016 |