Green Chemistry: A design framework for sustainability
Evan S.
Beach
,
Zheng
Cui
and
Paul T.
Anastas
*
Center for Green Chemistry and Green Engineering, Yale University, 225 Prospect Street, New Haven, CT 06511, United States. E-mail: paul.anastas@yale.edu; Fax: +1 203 436 8574; Tel: +1 203 432 5215
First published on 9th July 2009
Abstract
In this review we will highlight some of the science that exemplifies the principles of Green Chemistry, in particular the efficient use of materials and energy, development of renewable resources, and design for reduced hazard. Examples are drawn from a diverse range of research fields including catalysis, alternative solvents, analytical chemistry, polymer science, and toxicology. While it is impossible for us to be comprehensive, as the worldwide proliferation of Green Chemistry research, industrial application, conferences, networks, and journals has led to a wealth of innovation, the review will attempt to illustrate how progress has been made toward solving the sustainability goals of the 21st century by engaging at the molecular level.
 Evan S. Beach Evan S. Beach | Dr Evan Beach joined the Center for Green Chemistry and Green Engineering at Yale in 2007. He received his BS and PhD in Chemistry from Carnegie Mellon University, where he researched environmentally benign catalytic oxidation systems for water cleaning applications. His current research focuses on polymers, fine chemicals, and other useful materials from renewable feedstocks, with an emphasis on productive use of byproducts generated in biofuel production. |
 Zheng Cui Zheng Cui | Dr Zheng Cui is a postdoctoral research associate in the Center for Green Chemistry and Green Engineering at Yale. She received her PhD in Materials Science from Beijing University of Aeronautics and Astronautics in 2008, where her thesis work consisted of synthesis and characterization of chitosan-based proton exchange membranes for fuel cell applications. Her current research interests include applications of chitosan, lignin, and other underutilized polymers of biological origin. |
 Paul T. Anastas Paul T. Anastas | Dr Paul Anastas is the Teresa and John Heinz III Professor in the Practice of Green Chemistry for the Environment at Yale University and serves as the Director of the Center for Green Chemistry and Green Engineering at Yale. Trained as a synthetic organic chemist, Dr Anastas received his PhD from Brandeis University. He is credited with establishing the field of green chemistry during his time working for the U.S. Environmental Protection Agency as the Chief of the Industrial Chemistry Branch and as the Director of the U.S. Green Chemistry Program. He has published widely on topics of science through sustainability. (Photo credit: Michael Marsland) |
Broader contextGreen Chemistry has enormous implications for energy and the environment. As the chemical industry currently accounts for a significant portion of energy consumed by the manufacturing sector (and its corresponding emissions of CO2), advances in catalysis and alternatives to conventional heating in chemical processes will play an important role in ensuring the sustainability of the chemical enterprise. Additionally, as the world shifts from fossil fuels to solar, wind, hydro, and other readily renewable sources of energy, the demand for infrastructure presents an opportunity to build materials and technologies based on renewable resources, using chemical feedstocks in an efficient and non-polluting manner. Over the last 20 years, researchers in the field of Green Chemistry have contributed new ideas and techniques to move toward practical solutions to critical sustainability issues. Success in the future will depend on connecting diverse scientific disciplines through a common thread: reducing environmental impacts through design at the molecular level. |
1. Introduction
Green Chemistry is the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances.1 The green chemistry approach seeks to redesign the materials that make up the basis of our society and our economy—including the materials that generate, store, and transport our energy—in ways that are benign for humans and the environment and possess intrinsic sustainability. The concepts and practice of Green Chemistry have developed over nearly 20 years into a globe-spanning endeavor aimed at meeting the “triple bottom line”—sustainability in economic, social, and environmental performance.2 Many challenges still lie ahead, and the solutions will be found not only in the discipline of chemistry but at its interfaces with engineering, physics, and biology.3 New developments in toxicology such as predictive toxicology and toxicogenomics are making it ever more possible to advance the most important concept in Green Chemistry: design.The aphorism “an ounce of prevention is worth a pound of cure” is at the heart of Principle 1 of the Twelve Principles of Green Chemistry, a comprehensive set of design guidelines that have guided Green Chemistry development for many years.4 The cost of handling, treating, and disposing hazardous chemicals is so high that it necessarily stifles innovation: funds must be diverted from research and development (scientific solutions) to hazard management (regulatory and political solutions, often). For example, in the United States, the government has estimated that 4% of manufacturing GDP is spent on compliance with regulations related to environmental, worker, and product safety.5 Reviews of chemical accidents show that while the chemical industry is safer than other manufacturing jobs, exposure controls can and do fail. The consequence is injury and death to workers, which could have been avoided by working with less hazardous chemistry.6 Impacts on human health and the environment from dispersal of hazardous waste are similarly grim. The United States Environmental Protection Agency Toxic Release Inventory Program, which only monitors 650 out of 78,000 chemicals in commerce—in one country—reported more than 60 kg of toxic chemicals per second were released directly to the air, land, and water in 2005. A glance at the Blacksmith Institute “World's Worst Polluted Places” shows the devastating costs of these releases, and the monumental cleanup problems that are faced as a result of the “treatment” rather than “prevention” approach. In Green Chemistry, prevention is the approach to risk reduction (eqn 1):
| Risk = f(hazard × exposure) | (1) |
By minimizing the hazard portion of the equation, using innocuous chemicals and processes, risk cannot increase spontaneously through circumstantial means—accidents, spills, or disposal. This review will show how Green Chemistry has been tremendously successful in devising ways to reduce pollution through synthetic efficiency, catalysis, and improvements in solvent technology. We will discuss alternative synthetic methods that have been applied to reduce energy consumption in the chemical industry, and we will survey bio-based feedstocks and materials that are decreasing our reliance on depleted fossil resources. Finally, we will consider the challenges that lie ahead in designing safer chemicals: the establishment of a comprehensive set of design principles and interdisciplinary cooperation to move toward routine consideration of hazards as molecular properties just as malleable to chemists as solubility, melting point, or color.
2. Material and energy efficiency
Several of the Twelve Principles of Green Chemistry aim to minimize hazard by eliminating waste:• incorporating all starting materials into a product
• avoiding protecting groups whenever possible
• favoring catalytic reagents
• using less solvent and separation agents
• reducing energy consumption
Waste reduction translates very readily into economic benefits—wasted reagents are wasted money, and material that is not converted into product or recycled is essentially paid for twice: once as a feedstock, and again as a waste to be disposed of. Alternative solvents and solvent-free reactions contribute to safer conditions for chemical workers, since many conventional solvents are volatile, flammable, or associated with acute and chronic health effects.7 Syntheses and processes that are carefully designed to eliminate waste streams make questions of environmental persistence and toxicity moot. Most of the Green Chemistry success stories of the last 20 years are related to advances in waste minimization.
This area of Green Chemistry has been aided by the development of metrics for assessing the potential environmental impact of a synthesis or process. For more than a century, chemists have relied on the concept of “yield” almost exclusively to gauge the success of a reaction or series of reactions. A significant advance in accounting for chemical waste was the concept of “atom economy”, outlined by Trost in 1991.8 Atom economy is a measure of how many atoms in a reagent are incorporated into the product during a chemical transformation. Addition reactions, for example cycloadditions or bromination of olefins, are completely atom economical, since no atoms are lost during the transformations. Substitution reactions result in partial loss of atoms since the leaving group necessarily becomes waste; Trost gives the example of methylene group transfer using methyltriphenylphosphonium bromide, in which only 14 out of 365 Daltons are utilized productively. Elimination reactions are intrinsically the least atom economical, since the starting materials only lose mass and all eliminated atoms become waste. Since atom economy does not account for solvents and separation agents, the “E-factor” proposed by Sheldon is a complementary metric that accounts for all raw materials consumed but not incorporated into the product.9,10 The E-factor reports the amount of unit waste produced per unit product, and ideally will equal zero. Table 1, Sheldon's breakdown of waste generation by chemical industry sector, highlights the need for improvements particularly in fine chemical and pharmaceutical manufacturing. The custom synthesis of biomolecules in the biotech sector may yield E-factors well over 100. E-factors for nanoparticle production vary greatly and some production methods can reach 100,000.11
| Industry sector | Production/metric tons year−1 | E-factor/kg waste kg product−1 |
|---|---|---|
| Oil refining | 106–108 | <0.1 |
| Bulk chemicals | 104–106 | <1–5 |
| Fine chemicals | 102–104 | 5–50 |
| Pharmaceuticals | 10–103 | 25–100 |
Additional metrics such as “reaction mass efficiency” and “carbon efficiency” have been successfully adopted by the pharmaceutical industry. The variety of metrics to choose from is not confusing, rather, it provides ways to express waste and efficiency information most usefully to chemists, engineers, or business managers.12
2.1 Catalysis
Excessive waste generation can be attributed in part to reliance on traditional stoichiometric reagents.13Reductions with metals or metal hydrides, and oxidations with metal oxides such as permanganate and dichromate necessarily generate stoichiometric amounts of metal waste. Numerous conventional organic transformations rely on mineral and Lewis acids or metal hydroxides, creating waste streams laden with inorganic salts. Advances in catalysis are rendering these classical methods obsolete. The greener alternatives include catalytic hydrogenation (H2), oxidation (O2 or H2O2), carbonylation, and hydroformylation. Transition metal catalysis has created a toolbox of low- or zero-waste carbon–carbon bond-forming chemistry. The power of catalysis to advance sustainable chemistry was recognized by the Royal Swedish Academy of Sciences in 2005, who in awarding the Nobel Prize in Chemistry to Chauvin, Grubbs, and Schrock, noted that the scientists' pioneering work in olefin metathesis was a “great step forward for green chemistry”.14The three most recent U.S. Presidential Green Chemistry Challenge Awards given to academic researchers by the United States Environmental Protection Agency honored breakthroughs in catalysis.15 The 2008 prize was awarded for improvements in Suzuki coupling—a popular method for building complex chemical structures through carbon–carbon bond formation. Early Suzuki couplings were waste intensive; a typical synthesis might require reaction of an aryl chloride with a Grignard reagent followed by addition of a trialkylborate ester, generating stoichiometric magnesium, chloride, and alkoxide waste (Fig. 1), in addition to any waste associated with preparing the chlorinated feedstock. The award-winning chemistry is halogen-free, based on thermal, catalytic activation of an aryl carbon–hydrogen bond. The only coproduct is hydrogen. The iridium catalysts are robust and selective, allowing some syntheses to be carried out in solvent-free conditions.16
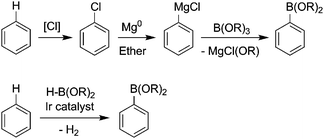 | ||
| Fig. 1 A green method for preparing boronic esters. The conventional route (top) results in stoichiometric magnesium, chloride, and alkoxide waste, while the new route (bottom) generates only hydrogen. | ||
The 2007 award recognized a powerful technique for enantioselective carbon–carbon bond formation; using a rhodium or iridium catalyst, an alkyne can be coupled to a carbonyl compound or imine with H2 acting as the mediator.17 The allyl alcohol or allyl amine products are valuable chiral compounds for production of pharmaceuticals and other fine chemicals. A conventional route to an allylamine might rely on a series of organometallic reagents, each generating stoichiometric metal waste. The new catalytic method completely incorporates the starting materials into the product molecule, with no byproducts (Fig. 2).
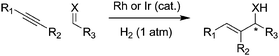 | ||
| Fig. 2 Hydrogen-mediated formation of carbon–carbon bonds, avoiding stoichiometric metal waste (X = NH, O). | ||
In 2006, the potential for catalysis to minimize another kind of waste—wasted energy—was highlighted. As biofuels have increased in popularity, the byproduct glycerol has flooded the market. Industry is seeking to develop a platform of valuable chemicals from glycerol, for example propylene glycol, which can be obtained by dehydration of glycerol and subsequent hydrogenation of the intermediate product (Fig. 3). The hydrogenation step typically requires hydrogen pressures in excess of 10 MPa and temperatures as high as 350 °C. A new process based on a recyclable copper–chromite catalyst requires just 1.4 MPa H2 and operates at 200 °C.18,19 As of mid-2007 a facility capable of producing 30 million kg year−1 was under construction.20
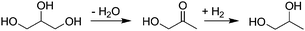 | ||
| Fig. 3 Reactive distillation and hydrogenation of glycerol to propylene glycol; recent improvements in the catalytic process allow the reaction to be carried out at significantly lower temperature and pressure. | ||
Green Chemistry has also aimed to eliminate waste caused by catalysts themselves, including metal, solvent, and auxiliary waste generated in catalyst quenching and separation processes. Many strategies have been developed to improve catalyst recyclability. Immobilization of catalysts on inorganic or polymer supports facilitates catalyst recovery and regeneration and minimizes contamination of products. Catalysts have also been designed with properties that allow them to be readily recovered by adjusting pH or temperature.21 Porous inorganic solids for use in heterogeneous catalysis are a good example of the green chemistry benefits that can be achieved using new techniques. These catalysts can be prepared with useful functionality (Brønsted- or Lewis-acidic or redox-active metal sites) and they can be designed so that the active sites are similar and well separated, avoiding the common problem in heterogeneous catalysis that different sites have a range of activities and selectivities.22 Solid “single-site” catalysts have been designed for shape-, regio-, and enantioselectivity, and they can be used to carry out chemical transformations in fewer steps than conventional processes and with significant reduction of waste byproducts.23 For example niacin (3-picolinic acid) and other useful oxygenated picolines are conventionally prepared with selenium dioxide, permanganate, or chromic acid in multistep, waste-intensive syntheses. In some cases 2.8 kg of inorganic waste is generated per kg niacin. But, a microporous aluminophosphate catalyst with Mn(III) sites converts 3-picoline to niacin in just one step without organic solvents under mild conditions (Fig. 4).24
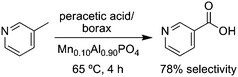 | ||
| Fig. 4 A microporous solid catalyst converts 3-picoline to niacin in a single step without heavy metal oxidants or organic solvents. | ||
Recyclable solid acid catalysts can eliminate hazardous waste associated with conventional Lewis acid catalysts such as AlCl3; industrially relevant applications include benzoylation of benzene with a sulfated zirconia catalyst.25 Mesoporous silica has been used to prepare a supported TEMPO catalyst that can oxidize alcohols in air without any transition metals (Fig. 5). The use of a TEMPO system avoids heavy metal oxidants, and the porous solid has allowed the catalysis to take place without transition metal promoters or high temperatures that are often required by TEMPO chemistry.26
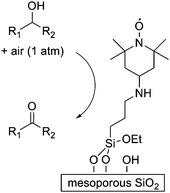 | ||
| Fig. 5 A supported TEMPO catalyst oxidizes alcohols to carbonyl compounds without any transition metals. The chemistry requires only catalytic amounts of NaNO2 and tetrabutylammonium bromide. | ||
Nature is a source of inspiration for new catalytic chemistry. Modeling of enzyme active sites has led to the development of first-row transition metal complexes with simple ligands that can carry out enzyme-like transformations. For example, non-heme iron oxygenases were the inspiration for the first iron catalysts to activate H2O2 to carry out stereospecific oxidation of alkanes (Fig. 6).27 The catalysts were further shown to enable epoxidation and cis-hydroxylation of alkenes.28 These kinds of oxidation reactions would ordinarily be carried out with stoichiometric organic peroxides or inorganic metal oxides, generating stoichiometric waste. Hydrogen peroxide, on the other hand, can generate only innocuous byproducts: water and oxygen. A similar bio-inspired iron catalyst was recently discovered that is capable of selectively oxidizing one of the five tertiary C–H bonds in artemisinin.29 In the case of complex molecules like artemisinin, selectivity usually comes at the price of additional synthetic steps to introduce or remove activating and protecting groups, each of which can add to hazardous waste streams. Ideally, further understanding of catalyst-derived selectivity will allow chemists to transform complex molecules in predictable ways, with a fraction of the environmental impact.30Iron complexes with tetraamidomacrocylic ligands (Fe–TAMLs) have been developed to activate hydrogen peroxide with activities and lifetimes comparable to peroxidase enzymes.31 These catalysts, which were the subject of a 1999 U.S. Presidential Green Chemistry Challenge Award, have found applications in a number of fields, most notably as an environmentally benign technology for cleaning endocrine disrupting chemicals from water.32,33
Biomimetic catalysis has also been a successful strategy in polymer synthesis. For example, hematin can be modified with poly(ethylene glycol) to create a “synthetic enzyme” that emulates horseradish peroxidase, catalyzing the synthesis of polyanilines, polyphenols, polypyrroles, and polythiophenes.34,35 Conventional routes to these materials can be waste-intensive; polyaniline synthesis often entails high temperature, inorganic oxidants, and toxic solvents, whereas enzymatic methods can be performed in water under mild conditions.36 The hematin-based catalyst retains the functionality of the peroxidase active site, but is stable to a wider range of reaction conditions. The bio-inspired synthesis can be used to produce useful materials including conducting polymers and non-toxic industrial antioxidants, with minimal solvent, chemical, and energy waste.37
It is often productive to take advantage of enzymes themselves to achieve selective transformations with a minimum of reagents and synthetic steps. For example, enzymatic synthesis of a precursor to the drug pregabalin eliminates the need for classical chiral resolution and multiple recrystallization steps, reducing waste streams by 80%.38 Enzymes can be used in tandem or in integration with chemical synthesis to prepare complex, chiral molecules from low-value starting materials.39 The reactions are not limited to water; useful enzymatic transformations have been carried out in neat organic solvents and ionic liquids, and of particular interest to green chemists are reactions in which enzymes facilitate transformations of undissolved substrates, including solid-to-solid chemistry.40 “BRENDA” (Braunschweig Enzyme Database, http://www.brenda-enzymes.info) is the world's largest compendium of enzyme data, manually collected from the primary literature, covering more than 4000 enzymes. The database is searchable by reaction types, substrates and products, industrial applications, structure, stability, and numerous other parameters.41
At the cutting edge of enzyme technology is directed evolution, a technique involving high-throughput screening of mutants. Directed evolution can make enzymes more broadly useful: it can improve stability; it can induce enzymes to accept unnatural substrates; it can change the reaction pathway so different products are obtained; or it can enhance the regio- or stereospecificity of the reactions.42 For example, in the synthesis of the pharmaceutical atorvastatin, an enzyme's performance was increased 4000-fold by introduction of 35 mutations. The enhanced enzymatic process meets a variety of green chemistry goals including reduced solvent, safer reagents, and elimination of wasteful purification steps.43
2.2 Alternative solvents
Solvents often account for the vast majority of mass wasted in syntheses and processes. Many conventional solvents are toxic, flammable, or corrosive. Their volatility contributes to air pollution, increases the risk of worker exposure, and has led to countless catastrophic accidents. Recovery and reuse, when possible, is often accomplished by energy-intensive distillation processes. Large proportions of solvent used, for example in pharmaceutical synthesis, contribute to hazardous waste streams, including contaminated water.44 Green chemists have made significant progress in reducing the negative impacts of solvent usage.45 Guides have been developed to suggest substitutions for the most problematic solvents.7,46 Non-toxic and non-flammable solvents like carbon dioxide and water have been explored in depth, as discussed below. Poly(ethylene glycols) are beginning to find use as safe, biodegradable solvents,47 and the frontiers of solvent research include “smart solvents” with switchable polarity that can reduce solvent waste and facilitate recycling.48It is often said that “the best solvent is no solvent”. The benefits of solvent-free chemistry often extend beyond waste reduction and hazard avoidance. There are numerous examples of shorter reaction times, and even enhanced activity and enantioselectivity of catalysts in the absence of solvent.49 More than 1000 preparative-scale solid–solid or solid–gas syntheses are known to give quantitative yield, and many are easily performed on the kilogram scale. In some cases, the corresponding solution chemistry is so difficult as to be practically impossible, for example formation of a 1 : 1 urea–glucose complex (Fig. 7).50
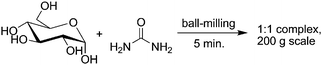 | ||
| Fig. 7 Solvent-free formation of a glucose–urea complex is achieved rapidly on a large scale, replacing a wasteful 6-month crystallization technique suitable only for milligram quantities. | ||
CO2 has been extensively studied as an environmentally benign solvent. It is abundant and inexpensive, often obtainable as a waste material from other processes. Its liquid and supercritical phases are accessible at mild temperature and pressure, and sensitivity of some reactions to easily tunable CO2 pressure gives chemists greater control over reaction rates and selectivity.51 The first “green” application of supercritical CO2 was replacement of the toxic solvent dichloromethane in decaffeination of coffee beans.52 CO2 remains a useful and safe solvent for routine extractions,53 but has also been developed as a reaction medium for synthesis of small molecules, polymers, and nanoparticles. There are numerous examples of homogeneous and heterogeneous catalysis carried out in CO2,51 and even biocatalysis is feasible.54Catalytic reactions in CO2 can be scaled up to industrially useful processes:55 the University of Nottingham has collaborated with Thomas Swan & Co. to construct a plant for conducting reactions on the scale of 100 kg h−1. The first reaction to be run was hydrogenation of isophorone to trimethylcyclohexanone over a palladium catalyst (Fig. 8).56 The recent invention of a CO2-based process for synthesizing H2O2 from H2 and O2 is a step toward making the environmentally friendly oxidant even “greener”; it reduces the risk of explosion and avoids the organic solvent and anthraquinone wastes generated by the conventional method.57 Progress in designing surfactants and stabilizers for CO2, particularly for water-in-CO2 microemulsions, will continue to expand the range of chemistry that can be performed in CO2 to include more ionic and polar reagents.58
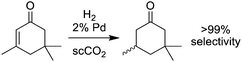 | ||
| Fig. 8 Industrial-scale hydrogenation of isophorone. In the process using supercritical CO2, no product purification steps are required. | ||
Water, perhaps the most innocuous substance on the planet, has also attracted much attention as an alternative to organic solvents, and it can be used for an enormous variety of chemistry including most types of carbon–carbon bond forming reactions as well as oxidations and reductions.59–61 Lewis acid catalysis, which is usually highly sensitive to trace moisture, can be carried out in aqueous conditions with careful selection of metals and ligands.62 Even dehydration reactions can be performed in water.63 In many of these cases, the aqueous chemistry avoids energy-intensive solvent purification and distillation steps associated with conventional solvents. However, the relatively low volatility of water may increase the energy needed to isolate products or remove contaminants from solution in some processes, making it necessary to always consider the overall environmental impact of the chemistry.
2.3 Green analytical chemistry
One of the twelve principles of green chemistry calls for improved monitoring of chemical syntheses and processes to prevent waste, reduce solvent and auxiliaries, and prevent formation of hazardous side products. This demands technologies that are real-time, placing limits on opportunities for chemical derivatization or instrument calibration and requiring that the technologies be adaptable to process equipment. A recent review discusses progress in the field, covering nanosensors, molecular recognition, and lab-on-a-chip capabilities that have made it possible to monitor heat, chemical concentrations, mass, and optical properties in-process.64 Microreactors (in which reaction components are manipulated in channels as small as 10 µm in diameter) are especially well suited for real-time, in-reactor monitoring because they allow exceptionally precise control of reagent concentrations and other reaction conditions in both time and space.65 For example, using in situ UV/visible or infrared spectroscopy monitoring, microreactor flow rates can be adjusted to optimize reaction yields.66Analytical methodologies themselves should also adhere to the principles of green chemistry. There is some irony in the fact that common methods for monitoring pollutants create dangerous waste streams; the chemical oxygen demand test used in wastewater analysis usually relies on carcinogenic hexavalent chromium as well as mercury and silver reagents.67 A recent review of the analytical chemistry literature has found significant improvements in “green” analytical methods. New techniques for extraction reduce solvent and energy demands by using ultrasound, microwaves, or supercritical fluids. Advances in membrane separations, solid-phase extraction, and flow injection analysis have reduced the environmental footprint of analytical techniques.53 Improvements in spectroscopy have made it possible to use research-grade instrumentation in-process or in the field. For example, NMR spectroscopy can be used to monitor flowing material, and low-field NMR techniques have been developed to unobtrusively measure moisture content, fat content, or fluorine content in solid materials without any kind of pretreatment, separation, or purification steps.68 Low-field NMR can determine the oil content in oilseeds at the rate of 20,000 seeds h−1, an analysis that would ordinarily be achieved by extraction with hazardous solvents.69 The National Environmental Methods Database maintained by the US Environmental Protection Agency and US Geological Survey (http://www.nemi.gov) has been updated to include “greenness” information in the method profiles, making it more convenient for analytical chemists to compare the environmental impacts of various techniques.53
2.4 Energy impacts
The chemical industry accounts for significant energy consumption and CO2 emissions.70 In the United States, the industry typically consumes 25–30% of the total energy used annually by the entire manufacturing sector, and energy use has been steadily increasing: in 2002 it was almost 1.5 PJ higher than 1991 (Fig. 9). The two categories of plastics manufacturing and “other” basic organic chemicals— including common monomers and solvents—represent more than half of the chemical industry energy use.71 Chemists often have more power over energy efficiency than chemical engineers or process engineers; if a green chemist devises a synthesis that avoids the need to accelerate a reaction with heat, control reactivity through cooling, or purify products through distillation, crystallization, or ultrafiltration, the potential energy savings are enormous.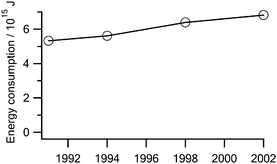 | ||
| Fig. 9 Annual energy consumption by the U.S. chemical industry, not including primary metals or coal and petroleum refinining. | ||
Alternative techniques like microwave-, sono-, or photo-assisted chemistry have been applied by green chemists in the last 20 years, taking advantage of advances in technology to not only save energy but also reduce reaction times, simplify experimental conditions, and increase the effectiveness of catalysts. In microwave chemistry, it is now possible to use commercial equipment to control all aspects of microwave heating, greatly improving safety and reproducibility. Microwaves enable very rapid heating and cooling, avoiding the need for prolonged residence time at high temperature.72 Energy savings compared to conventional heating depends on efficiency in converting electric energy to microwave energy, as well as the characteristics of the reactor, the amount of reaction mixture, and the capability of the reaction components to absorb microwave energy.73 The use of microwave heating on an industrial scale is in early stages of research, though so far it is favorable in comparison to conventional heating; for example, a continuous microwave reactor has been applied in biodiesel production on the scale of L min−1.74 Also at the forefront of microwave research is using microwave energy to support other “green” techniques such as solvent-free or aqueous chemistry and reactions using recyclable catalysts and supports.75
Sonochemistry is the use of high frequency (20–100 kHz) sound waves to promote chemical reactions. The collapse of bubbles formed in solution by the sound waves results in very high temperature and pressure, and potentially an order of magnitude more energy available per molecule than thermal heating.76 Unlike microwaves, sonochemistry can break chemical bonds and access novel reaction pathways. Sound waves can also lead to “false sonochemistry”: effects analogous to high-speed stirring that can also improve the efficiency of chemical reactions.77 There are many examples of industrial-scale reactions promoted by sonochemistry with increased yields in shorter amounts of time than conventional heating. It cannot always be assumed that sonochemistry will save energy, though in some cases, for example methyl transesterification of triglycerides, it is an order of magnitude more efficient in terms of mass of product per energy consumed.78 Other methods of promoting bubble collapse in solution are potentially far more energy efficient: hydrodynamic cavitation, in which turbulent flow is used to create bubbles, also promotes chemical reactions.79 In the same transesterification study mentioned above, hydrodynamic cavitation was more efficient than conventional heating by a factor of 1000.
Photochemistry also offers alternatives to thermal processes. Photons, unlike ordinary activating agents or catalysts, leave no trace after they are used. In many cases, the molecular transformations achieved would require additional synthetic steps.80 Absorption of photons does not necessarily depend on solvent, so photochemistry expands the range of possible solid-to-solid chemical reactions.81 Sunlight—the most abundant source of energy on the planet—can be used to produce useful chemicals on the multi-kilogram scale. Using photoreactors in Germany, researchers have carried out photoacylation and photooxygenations, producing important synthetic intermediates and commercial fragrances in bulk with minimal consumption of fossil energy.82 Another example of the energy savings that can be realized by harnessing sunlight or artificial UV light is photo-curable resins: BASF has developed automotive primer coatings that crosslink readily under light irradiation. The new coatings obviate the need for energy-intensive bake ovens used to thermally cure conventional primers.37 Because chemically important UV radiation only accounts for a small percentage of solar photons reaching the earth's surface, continuing development of visible-light-absorbing sensitizers will promote more effective use of natural sunlight. Mimicry of photosynthesis, using synthetic systems to collect light and convert the energy into a form that can power enzymatic reactions, may soon revolutionize the way energy is generated and used in chemical manufacturing.83
Green chemistry also has much to gain from “noncovalent derivatization” techniques. Whereas chemists have traditionally driven reactions through enthalpy—covalent bond-breaking and bond-making reactions—nature is usually reliant on entropic processes, with no need for high temperature conditions. Chemists can manipulate the physical properties of materials like melting point, solubility, vapor pressure, and diffusivity using specific noncovalent interactions including hydrogen bonding, π-stacking, lipophilic–lipophilic interactions, and electrostatic interactions. While each interaction is low in energy, accumulation of interactions eventually causes a system to reach a “tipping point” where a dramatic shift from one state to another suddenly occurs.84,85 Predicting and controlling this process can potentially eliminate pollution associated with solvents and purification, and should require only minimal energy input. Noncovalent chemistry has been used to control the stability of crystalline complexes in Polaroid Instant Photography.86
3. Renewable resources
One of the major goals of green chemistry is to produce chemical feedstocks sustainably, from annually renewable resources instead of petroleum. Nature produces biomass on the scale of about 180 billion metric tons year−1, of which only about 4% is currently utilized by humans. Most (about 75%) is in the form of carbohydrates, about 20% is lignin, and the remainder includes fats, proteins, and terpenes.87Currently, the major nonfood uses of carbohydrates are production of ethanol, furfural, sweeteners, lactic acid, surfactants, and pharmaceuticals (e.g.penicillin).88 However, routes are available for converting low-value carbohydrates into a much wider range of industrially useful products. Simple carbohydrates derived from starch, hemicelluloses, and cellulose can be converted into “building block” compounds via biological or chemical conversion. The US Department of Energy and Pacific Northwest National Laboratory have identified 12 of these intermediate chemicals that could be further exploited to produce more than 300 small molecules, many of which are commercially valuable feedstocks currently derived from petroleum feedstocks.89 Microbial conversion of glucose and other monosaccharides to 3-dehydroshikimic acid provides routes to useful aromatic compounds including vanillin and catechols.90
Lignin, one of the main structural components of plants and a byproduct of the pulp and paper industry, is nature's richest source of aromatic carbon. Since lignin is a high molecular weight, irregular macromolecule, production of small molecules containing aromatic rings will require advances in catalysis to become feasible on a large scale. One principle of Green Engineering is “conserve complexity”:91lignin has natural complexity that would be extraordinarily time-, material-, and energy-intensive to reconstruct from smaller molecules. It may be most productive to seek uses of lignin where its macromolecular structure is beneficial. According to the US Department of Energy, this is where the lowest-risk gains are to be made in lignin research. Macromolecular lignin could be potentially used to make high-strength, lightweight carbon fibers to replace steel. Other areas of opportunity include polymer additives, resins, adhesives, and binders.92 No matter the molecular weight range, a platform of lignin chemicals will make the abundant natural material much more valuable than it currently is as a combustible waste.
Increasingly, natural oils have been used instead of petrochemical- or silicon-based feedstocks to develop polymers, lubricants, surfactants, and emulsifiers. This sustainable oleochemistry currently faces challenges because many vegetable oils are also in high demand for energy and food uses.93 However, the increasing popularity of “second-generation” biofuel crops like Jatropha curcas,94fuel from cellulosic biomass,95 and production of oils from cultivation of algae96 are all expected to remedy supply concerns. Waste oil, like cashew nut shell liquid from the cashew nut-processing industry, is also a “green” starting material. Glycolipids synthesized from the nut shell liquid can be applied in soft materials, biosensors, liquid crystal displays, and pharmaceutical delivery.97
Soy and chickens are among the sources of proteins used productively by green chemists. Soy flour can be chemically modified to create an adhesive that mimics the water-resistant natural glue mussels use to attach to rocks. The new soy adhesive is an alternative to urea–formaldehyde, the binder used in plywood boards, and has already replaced millions of kg of the toxic synthetic resin.98 Chicken farms produce waste feathers on the scale of billons of kg year−1. The feathers, which are more than 90% keratin, can be carbonized to create strong, lightweight fibers with properties suitable for electronic circuitry. The Affordable Composites from Renewable Sources (ACRES) group at the University of Delaware combines the feather fibers with soy-based resins to produce biodegradable circuit boards. The feather–soy composites may also prove to be useful structural materials for car parts and building construction.99
The use of CO2 as a renewable source of carbon has been recently reviewed.100 While this application of CO2 as a raw material is not expected to significantly reduce levels of CO2 in the atmosphere, the nontoxic, non-flammable gas is still considered to be an environmentally friendly reagent. So far the most successful use of CO2 as a feedstock has been in the formation of carbonates. Dimethyl carbonate is an alternative to the toxic chemical phosgene, used in polycarbonate and polyurethane synthesis.101 Ironically, dimethyl carbonate itself is ordinarily synthesized from phosgene as well, but new syntheses are based on CO2.102 Cyclic carbonates, useful feedstocks for polycarbonate synthesis, can be prepared from epoxides or directly from alkenes. The synthesis from alkenes has additional “green” characteristics: it is performed in water without use of metal catalysts.103 The preparation of polycarbonates from CO2 has been enabled by the invention of highly active polymerization catalysts. Among these CO2-based polymers, poly(cyclohexene carbonate) shows promise as an alternative to polystyrene.104 CO2 has also been used in cement–fiber composites. The process consumes waste CO2, uses little energy, and offers alternatives to construction materials that are prepared by CO2-emitting processes.105
Commercial plastics are mostly prepared from fossil fuel feedstocks, accounting for a significant portion (approx. 5%) of annual petroleum production.106 Increasing pressures on supplies of fossil fuels, toxicity of commonly used monomers, and polymer persistence in the environment are all concerns that may be addressed by invention of new polymer materials based on biological feedstocks. Polysaccharides have been used as a platform for plastic materials for many decades: chemically modified cellulose, starches, xanthan, and pullulan are employed in a variety of applications.107 Chitin is one of the most abundant biopolymers but also one of the most underutilized; 11 billion metric tons are generated each year in the biosphere, but the commercial market is on the order of 5000 metric tons. Chitosan (deacetylated chitin) and its derivatives have already found applications as ingredients in cosmetics and personal care products, adsorbents for wastewater treatment, controlled-release agents, artificial human tissue, and biodegradable food packaging.108,109 The ease with which chitin and chitosan structures can be modified should create many opportunities for chemists to devise new bio-based polymers with a wide range of functional groups and mechanical properties.110
Polyester materials like polyhydroxylalkanoates (PHAs) and poly(lactic acid) (PLA) are at present among the most commercially successful renewable, biodegradable polymers. PHAs have been commercialized by Metabolix and Archer Daniel Midlands Co., using genetically engineered microbes to synthesize the polymer from fermentable raw materials.37 A facility is under construction that will be capable of producing 50 million kg year−1 of PHA bioplastic.111PHA can also be produced by genetically engineered switchgrass, accounting for nearly to 4% of the dry weight of the leaves. Thus, as biofuel-from-switchgrass projects expand, PHAs are poised to become more prevalent.112 Large-scale production of PLA was pioneered by NatureWorks, now jointly owned by Cargill and Teijin Ltd. of Japan. The production process has been honored with a Presidential Green Chemistry Challenge Award for following all Twelve Principles of Green Chemistry: its avoids organic solvent, uses efficient catalysis to achieve high yields, and reduces waste through recycle streams. The process uses up to 50% less petroleum resources compared to conventional polymers.113 Lactic acid is conventionally derived from corn sugar but it can also be obtained by fermentation of cellulosic waste.114PLA may find a broader range of applications as progress is made in developing adequate plasticizers.115 Preparation of biocomposite materials from PLA and agricultural wastes offers an alternative to non-degradable, difficult-to-recycle petroleum-based resins.116,117
Optimization of the synthetic pathways to bio-based feedstocks and invention of new bio-based materials will reduce our reliance on depleted petroleum resources and support the production of fuels by biorefineries. Integrated biorefineries, in which energy, chemicals, and food processing are combined, will be crucial to extracting the maximum value from abundant biomass and meeting the sustainability goals of the next 100 years.118 It has been suggested that biorefineries and petroleum refineries can operate synergistically as well; with design of more highly selective catalysts for hydrotreating and catalytic cracking of bio-based feedstocks to produce alkane, olefin, and aromatic fuels, existing infrastructure might be used to smooth the transition to a sustainable economy.119 In the near future, biomass will only be a partial solution to the challenge of sustainable energy: a study by the Oak Ridge National Laboratory has noted that in the United States, for example, renewable sources of biomass could potentially account for 30% of current petroleum consumption by 2030. Thus we must rely on other renewable energy technology as well, ideally solar power.120 However, biomass is our only non-depleting source of chemical feedstocks. Invention and application of bio-based materials has advanced tremendously in the last 20 years, and this will continue to be an area of research with many opportunities for green chemists.
4. Designing safer chemicals and processes
Increasingly chemists have access to more information about the chemicals and classes of chemicals that threaten human and environmental health. It is relatively easy, conceptually, to eliminate known hazards from a synthesis or process, but it is much more difficult to be sure that the alternative will be benign. The future of green chemistry requires that chemists take on a more proactive role in designing for reduced hazard, to avoid the kind of cycle that leads to accidental environmental damage, toxicity surprises, and daunting cleanup problems:1. A chemist synthesizes a novel chemical.
2. The chemical enters commerce and is dispersed around the world.
3. Alarms are sounded about persistence, bioaccumulation, toxicity, etc.
4. Legislation bans or restricts further use; remediation programs are implemented.
5. A novel chemical is required to replace the previous offender.
The development and routine implementation of “green” design principles will be crucial in the coming years, as green chemists grapple with the phenomena of endocrine disruption, synergism, and non-linear dose-response curves that contradict the ancient notion that “the dose makes the poison”.121,122 Toxicity concerns about the negative effects of ubiquitous chemicals like organochlorine compounds,123perfluorooctanoic acid,124 polybrominated flame retardants,125phthalate plasticizers,126 and bisphenol A127 ensure that chemists will be pressed to improve the safety of existing, well-established technologies and to guarantee that emerging new fields like nanotechnology are not derailed by safety concerns.11,128 It will be necessary for chemists to relate molecular structure not only to intended function, but also unintended behavior: mobility, persistence, and fate in humans, animals, and the environment. It has been proposed that design rules should be established based on (in order of importance):129,130
1. Molecular toxicology that relates structural features to mechanism of action.
a. Avoidance of toxic chemical classes or functional groups.
b. Structural blocking or relocation of toxic groups.
2. Quantitative structure-activity relationships (QSARs) that predict potential hazard when mechanistic data is unavailable.
3. Toxicokinetics and toxicodynamics studies to ensure safe metabolism.
a. Planned biochemical elimination of toxic groups.
b. Facilitation of excretion.
c. Facilitation of biodegradation.
4. Decreasing bioavailability so that a chemical released into the environment cannot pass through relevant biological barriers.
a. Appropriate molecular size.
b. Low volatility.
c. Appropriate water solubility and lipophilicity.
d. Consideration of specific routes of absorption.
DeVito has extensively reviewed many of the design elements that are part of this framework.131 His valuable compendium of chemical design information includes:
• A catalog of electrophilic functional groups, the biological nucleophiles they may react with, and the toxic effects.
• Examples of how molecular toxicology and QSARs have led to design rules for less hazardous carboxylic acids, nonylphenols, and other industrially important chemicals.
• Isosteric replacements (groups of atoms that have similar characteristics but are not necessarily structurally related).
• Examples of design for safe metabolism.
At present, some of the most thoroughly laid-out design rules for designing safer chemicals are related to biodegradability. If a chemical does not persist beyond the end of its useful function, it has reduced chance of exerting toxic effects on humans or lifeforms we depend on. Small molecule biodegradability has been recently reviewed by Boethling et al., who outline the guidelines that can be inferred from structure-degradability relationships, provide examples related to industrial chemistry, and survey software and database resources.132 With the exception of natural polymers and polymers derived from renewable resources, most high-molecular-weight molecules are extremely resistant to microbial action. In some cases it may be possible to impart degradability to conventional petroleum-based polymers by derivatizing them with degradable functional groups,133 but the problem of persistent plastics remains a significant challenge.134
Computational methods for exploring the properties of molecules have become highly sophisticated. In particular, the refinement of models for drug discovery and protein-small molecule interactions is creating powerful tools for pharmaceutical research—and potentially green chemists. The ability to predict the “druglikeness” of molecules allows one to determine if a molecule has favorable ADME (absorption, distribution, metabolism, and excretion) characteristics, and gain information about blood-brain barrier permeability, cell permeability, solubility, and likely metabolites.135 A chemist designing, for example, plasticizers for use in baby bottles might be able to use these capabilities to prescreen candidate molecules and reject structures with high bioavailability. One new tool for screening potentially hazardous chemicals is “Shape Signatures”, an algorithm that encodes molecular shape and polarity characteristics into simple, easy-to-compare metrics. Shape Signatures has been applied to high-quality protein–ligand data contained in the public Protein Data Bank, allowing chemists to screen molecules for potential biological receptors.136 The technique has also been used to identify novel estrogen antagonists based on similarities to known estrogens or anti-estrogens, showing that it will be a useful tool for identifying potential endocrine-disrupting chemicals.137
The growing concerns about pharmaceuticals in the environment and the animal testing that is being carried out in response may provide useful design guidelines to green chemists. Many drug receptors are more highly conserved between mammals and fish than between mammals and Daphnia or algae.138 Mammalian data from pharmaceutical testing can been applied to identify drugs that may elicit chronic toxicity in fish.139 It is also useful to run these studies in the reverse direction: using fish to screen non-pharmaceutical chemicals for potential bioactivity in humans. Zebrafish (Danio rerio) in particular have shown tremendous promise as a way to accurately model human diseases; zebrafish assays have been used to screen small molecules for bioactivity including pharmaceutical properties or mutagenicity.140,141 Using fish, Daphnia, algae, and bacterial testing more routinely, not only in the pharmaceutical industry, will help avert environmental disasters and build chemists' knowledge of toxic mechanisms of action.
The future of designing safer chemicals will most likely be linked to developments in toxicogenomics and other “omics” fields. Toxicogenomics is the study of the effect of a toxin on the expression of genes on the cell level. The patterns of gene expression offer “fingerprints” for different kinds of toxicological responses, allowing chemicals to be classed according to the kind of biological damage they are capable of. Toxicogenomic information can be supplemented by proteomics (patterns of protein expression) and metabolomics/metabonomics (changes in levels of metabolites).142 Epigenetics, the study of heritable changes in gene expression that occur without changes in DNA sequence, will also contribute to our knowledge of mechanisms of action.143 Together, these fields that lie at the interface of toxicology, biology, and medicine will generate a corpus of information about specific hazards that can be linked to specific chemicals. Elucidating design principles from “omics” data will be challenging, but might offer the greatest breakthroughs for green chemists working at the cutting edge.
5. Future challenges and opportunities
The brief history of the field of Green Chemistry is marked with extraordinary creativity and accomplishments in achieving the dual goal of merging superior environmental and economic performance. This has generally been accomplished, as shown above, through the important tactic of improving a single important element or characteristic such as toxicity, persistence, or energy consumption. The powerful reality that is beginning to be realized and that must be exploited in the future is that the Principles of Green Chemistry can be approached as a unified system. Rather than thinking of the principles as isolated parameters to be optimized separately, one can view the principles as a cohesive system with mutually reinforcing components.This approach will be particularly important as we strive to understand the fundamentals of sustainability. While many of the current approaches seek to address important elements of sustainability, e.g., energy, or water, or food, it is important to recognize that all of these elements of sustainability are inextricably linked. Therefore, one important strategy will be to address these interconnected issues at the place where they all intersect: the molecular level. While no one would argue that this makes the challenges easy, it does become conceptually more straightforward through the principles of Green Chemistry.
References
- P. T. Anastas, L. G. Heine and T. C. Williamson, in Green Chemical Syntheses and Processes, ed. P. T. Anastas, L. G. Heine and T. C. Williamson, American Chemical Society, Washington, DC, 2000, pp. 1–6 Search PubMed.
- J. Elkington, Calif. Manage. Rev., 1994, 36, 90–100 Search PubMed.
- I. T. Horvath and P. T. Anastas, Chem. Rev., 2007, 107, 2169–2173 CrossRef CAS.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- Manufacturing in America: A Comprehensive Strategy to Address the Challenges to U.S. Manufacturers, U.S. Department of Commerce, 2004 Search PubMed.
- A. S. Matlack, in Introduction to Green Chemistry, CRC Press, Boca Raton, FL, 2001, pp. 6–25 Search PubMed.
- A. D. Curzons, D. C. Constable and V. L. Cunningham, Clean Prod. Process., 1999, 1, 82–90.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- R. A. Sheldon, Chem. Ind. (London), 1992, 903–906 CAS.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- R. A. Sheldon, Chem. Commun., 2008, 3352–3365 RSC.
- Press Release: The Nobel Prize in Chemistry 2005, http://nobelprize.org/nobel_prizes/chemistry/laureates/2005/press.html, accessed 3/18/2008 Search PubMed.
- United States Environmental Protection Agency: The Presidential Green Chemistry Challenge Award Recipients 1996–2008, http://www.epa.gov/gcc/pubs/docs/award_recipients_1996_2008.pdf, accessed 8/7/2008 Search PubMed.
- J.-Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka, Jr. and M. R. Smith, III, Science, 2002, 295, 305–308 CrossRef CAS.
- E. Skucas, M.-Y. Ngai, V. Komanduri and M. J. Krische, Acc. Chem. Res., 2007, 40, 1394–1401 CrossRef CAS.
- C.-W. Chiu, M. A. Dasari, G. J. Suppes and W. R. Sutterlin, AIChE J., 2006, 52, 3543–3548 CrossRef CAS.
- M. A. Dasari, P.-P. Kiatsimkul, W. R. Sutterlin and G. J. Suppes, Appl. Catal., A, 2005, 281, 225–231 CrossRef CAS.
- S. Shelley, Chem. Eng. Prog., 2007, 103, 6–9 Search PubMed.
- J. A. Gladysz, Chem. Rev., 2002, 102, 3215–3216 CrossRef CAS.
- J. M. Thomas and R. Raja, Top. Catal., 2006, 40, 3–17 CrossRef CAS.
- J. M. Thomas and R. Raja, Acc. Chem. Res., 2008, 41, 708–720 CrossRef CAS.
- R. Raja, J. M. Thomas, M. Greenhill-Hooper, S. V. Ley and F. A. A. Paz, Chem.–Eur. J., 2008, 14, 2340–2348 CrossRef CAS.
- J. H. Clark, Acc. Chem. Res., 2002, 35, 791–797 CrossRef CAS.
- B. Karimi, A. Biglari, J. H. Clark and V. Budarin, Angew. Chem., Int. Ed., 2007, 46, 7210–7213 CrossRef CAS.
- C. Kim, K. Chen, J. Kim and L. Que, Jr., J. Am. Chem. Soc., 1997, 119, 5964–5965 CrossRef CAS.
- K. Chen, M. Costas, J. Kim, A. K. Tipton and L. Que, Jr., J. Am. Chem. Soc., 2002, 124, 3026–3035 CrossRef CAS.
- M. S. Chen and M. C. White, Science, 2007, 318, 783–787 CrossRef CAS.
- R. H. Crabtree, Science, 2007, 318, 756–757 CrossRef CAS.
- A. Ghosh, D. A. Mitchell, A. Chanda, A. D. Ryabov, D. L. Popescu, E. C. Upham, G. J. Collins and T. J. Collins, J. Am. Chem. Soc., 2008, 130, 15116–15126 CrossRef CAS.
- S. Sen Gupta, M. Stadler, C. A. Noser, A. Ghosh, B. Steinhoff, D. Lenoir, C. P. Horwitz, K.-W. Schramm and T. J. Collins, Science, 2002, 296, 326–328 CrossRef.
- N. W. Shappell, M. A. Vrabel, P. J. Madsen, G. Harrington, L. O. Billey, H. Hakk, G. L. Larsen, E. S. Beach, C. P. Horwitz, K. Ro, P. G. Hunt and T. J. Collins, Environ. Sci. Technol., 2008, 42, 1296–1300 CrossRef CAS.
- F. F. Bruno, R. Nagarajan, S. Roy, J. Kumar, S. Tripathy and L. Samuelson, Mater. Res. Soc. Symp. Proc., 2001, 660(JJ8.6), 1–6.
- F. F. Bruno, S. A. Fossey, S. Nagarajan, R. Nagarajan, J. Kumar and L. A. Samuelson, Biomacromolecules, 2006, 7, 586–589 CrossRef CAS.
- P. Xu, A. Singh and D. L. Kaplan, Adv. Polym. Sci., 2006, 194, 69–94 CAS.
- The Presidential Green Chemistry Challenge Awards Program: Summary of 2005 Award Entries and Recipients, http://epa.gov/greenchemistry/pubs/docs/award_entries_and_recipients2005.pdf, accessed 8/8/2008 Search PubMed.
- C. A. Martinez, S. Hu, Y. Dumond, J. Tao, P. Kelleher and L. Tully, Org. Process Res. Dev., 2008, 12, 392–398 Search PubMed.
- A. Bruggink, R. Schoevaart and T. Kieboom, Org. Process Res. Dev., 2003, 7, 622–640 Search PubMed.
- A. Basso, S. Cantone, C. Ebert, P. J. Halling and L. Gardossi, in Organic Synthesis with Enzymes in Non-Aqueous Media, ed. G. Carrea and S. Riva, Wiley-VCH, Weinheim, Germany, 2008, pp. 279–301 Search PubMed.
- J. Barthelmes, C. Ebeling, A. Chang, I. Schomburg and D. Schomburg, Nucleic Acids Res., 2007, 35, D511–D514 CrossRef CAS.
- M. T. Reetz, in Adv. Catal., Elsevier, Inc., San Diego, CA, 2006, pp. 1–69 Search PubMed.
- R. J. Fox, S. C. Davis, E. C. Mundorff, L. M. Newman, V. Gavrilovic, S. K. Ma, L. M. Chung, C. Ching, S. Tam, S. Muley, J. Grate, J. Gruber, J. C. Whitman, R. A. Sheldon and G. W. Huisman, Nat. Biotechnol., 2007, 25, 338–344 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2005, 7, 267–278 RSC.
- P. T. Anastas, in Clean Solvents: Alternative Media for Chemical Reactions and Processing, ed. M. A. Abraham and L. Moens, Oxford University Press, 2002, pp. 1–9 Search PubMed.
- C. Jimenez-Gonzalez, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Clean Technol. Environ. Policy, 2005, 7, 42–50 Search PubMed.
- J. Chen, S. K. Spear, J. G. Huddleston and R. D. Rogers, Green Chem., 2005, 7, 64–82 RSC.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS.
- P. J. Walsh, H. Li and C. A. de Parrodi, Chem. Rev., 2007, 107, 2503–2545 CrossRef CAS.
- G. Kaupp, CrystEngComm, 2006, 8, 794–804 RSC.
- W. Leitner, Acc. Chem. Res., 2002, 35, 746–756 CrossRef CAS.
- K. Zosel, Angew. Chem., Int. Ed., 1978, 17, 702–709 CrossRef.
- L. H. Keith, L. U. Gron and J. L. Young, Chem. Rev., 2007, 107, 2695–2708 CrossRef CAS.
- T. Matsuda, T. Harada and K. Nakamura, Green Chem., 2004, 6, 440–444 RSC.
- P. Licence and M. Poliakoff, in Multiphase Homogeneous Catalysis, ed. B. Cornils, W. A. Herrmann, I. T. Horvath, W. Leitner, S. Mecking, H. Olivier-Bourbigou and D. Vogt, 2005, pp. 734–746 Search PubMed.
- P. Licence, J. Ke, M. Sokolova, S. K. Ross and M. Poliakoff, Green Chem., 2003, 5, 99–104 RSC.
- Q. Chen and E. J. Beckman, Green Chem., 2007, 9, 802–808 RSC.
- J. Eastoe and S. Gold, Phys. Chem. Chem. Phys., 2005, 7, 1352–1362 RSC.
- C.-J. Li, Chem. Rev., 1993, 93, 2023–2035 CrossRef CAS.
- C.-J. Li, Chem. Rev., 2005, 105, 3095–3165 CrossRef CAS.
- C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68–82 RSC.
- S. Kobayashi and C. Ogawa, Chem.–Eur. J., 2006, 12, 5954–5960 CrossRef CAS.
- K. Manabe, S. Iimura, X.-M. Sun and S. Kobayashi, J. Am. Chem. Soc., 2002, 124, 11971–11978 CrossRef CAS.
- C. M. A. Brett, Pure Appl. Chem., 2007, 79, 1969–1980 CrossRef CAS.
- S. J. Haswell and P. Watts, Green Chem., 2003, 5, 240–249 RSC.
- B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300–2318 CrossRef.
- D. G. Miller, S. V. Brayton and W. T. Boyles, Water Environ. Res., 2001, 73, 63–71 CrossRef CAS.
- A. Nordon, C. A. McGill and D. Littlejohn, Analyst, 2001, 126, 260–272 RSC.
- L. A. Colnago, M. Engelsberg, A. A. Souza and L. L. Barbosa, Anal. Chem., 2007, 79, 1271–1274 CrossRef CAS.
- Energy Use and Energy Intensity of the U.S. Chemical Industry, Energy Analysis Department, Environmental Energy Technologies Division, Ernest Orlando Lawrence Berkeley National Laboratory, 2000 Search PubMed.
- Manufacturing Energy Consumption Survey: Manufacturing and Industrial Energy Uses and Costs, Energy Information Administration, United States Department of Energy, 1991–2002 Search PubMed.
- C. R. Strauss and R. S. Varma, Top. Curr. Chem., 2006, 266, 199–231 CAS.
- M. Nüchter, B. Ondruschka, W. Bonrath and A. Gum, Green Chem., 2004, 6, 128–141 RSC.
- N. E. Leadbeater, T. M. Barnard and L. M. Stencel, Energy Fuels, 2008, 22, 2005–2008 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629–639 CrossRef CAS.
- K. S. Suslick and D. J. Flannigan, Annu. Rev. Phys. Chem., 2008, 59, 659–683 CrossRef CAS.
- G. Cravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180–196 RSC.
- P. R. Gogate, Chem. Eng. Process., 2008, 47, 515–527 CrossRef CAS.
- K. S. Suslick, M. M. Mdleleni and J. T. Ries, J. Am. Chem. Soc., 1997, 119, 9303–9304 CrossRef CAS.
- S. Protti, D. Dondi, M. Fagnoni and A. Albini, Pure Appl. Chem., 2007, 79, 1929–1938 CrossRef CAS.
- A. Natarajan, D. Ng, Z. Yang and A. Garcia-Garibay Miguel, Angew. Chem., Int. Ed., 2007, 46, 6485–6487 CrossRef CAS.
- M. Oelgemöller, C. Jung and J. Mattay, Pure Appl. Chem., 2007, 79, 1939–1947 CrossRef CAS.
- D. Gust, D. Kramer, A. Moore, T. A. Moore and W. Vermaas, MRS Bull., 2008, 33, 383–387 CAS.
- J. C. Warner, Pure Appl. Chem., 2006, 78, 2035–2041 CrossRef CAS.
- S. Trakhtenberg and J. C. Warner, Chem. Rev., 2007, 107, 2174–2182 CrossRef CAS.
- J. C. Warner, in Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, ed. P. T. Anastas and T. C. Williamson, Oxford University Press, New York, NY, 1998, pp. 336–346 Search PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS.
- F. W. Lichtenthaler, in Methods and Reagents for Green Chemistry: An Introduction, ed. P. Tundo, A. Perosa and F. Zecchini, Wiley, Hoboken, NJ, 2007, pp. 23–63 Search PubMed.
- Top Value Added Chemicals from Biomass, Volume I– Results of Screening for Potential Candidates from Sugars and Synthetic Gas, Pacific Northwest National Laboratory, National Renewable Energy Laboratory, Office of Biomass Program, 2004 Search PubMed.
- K. M. Draths, S. Kambourakis, K. Li and J. W. Frost, ACS Symp. Ser., 2001, 784, 133–146 CAS.
- P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 95A–101A CAS.
- Top Value Added Chemicals from Biomass, Volume II– Results of Screening for Potential Candidates from Biorefinery Lignin, Pacific Northwest National Laboratory and the National Renewable Energy Laboratory, 2007 Search PubMed.
- K. Hill, Pure Appl. Chem., 2007, 79, 1999–2011 CrossRef CAS.
- G. M. Gübitz, M. Mittelbach and M. Trabi, Bioresour. Technol., 1998, 67, 73–82.
- R. D. Perlack, L. L. Wright, A. F. Turhollow, R. L. Graham, B. J. Stokes and D. C. Erbach, Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion Ton Annual Supply (Oak Ridge National Laboratory, Oak Ridge, TN), http://feedstockreview.ornl.gov/pdf/billion_ton_vision.pdf, accessed 8/10/2008 Search PubMed.
- Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert and A. Darzins, Plant J., 2008, 54, 621–639 CrossRef CAS.
- P. K. Vemula and G. John, Acc. Chem. Res., 2008, 41, 769–782 CrossRef CAS.
- The Presidential Green Chemistry Challenge Awards Program: Summary of 2007 Award Entries and Recipients, http://www.epa.gov/greenchemistry/pubs/docs/award_entries_and_recipients2007.pdf, accessed 8/14/2008 Search PubMed.
- R. P. Wool and X. S. Sun, Bio-based polymers and composites, Elsevier Academic Press, Boston, 2005 Search PubMed.
- T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706–716 CrossRef CAS.
- B. M. Bhanage, S.-i. Fujita, Y. Ikushima, K. Torii and M. Arai, Green Chem., 2003, 5, 71–75 RSC.
- N. Eghbali and C.-J. Li, Green Chem., 2007, 9, 213–215 RSC.
- S. D. Allen, C. M. Byrne and G. W. Coates, in Feedstocks for the Future, ed. J. J. Bozell and M. K. Patel, American Chemical Society, Washington, DC, 2006, pp. 116–129 Search PubMed.
- R. Jones, in Green Engineering, ed. P. T. Anastas, L. G. Heine and T. C. Williamson, American Chemical Society, Washington, DC, 2001 Search PubMed.
- J. Baldwin, in Chemical Aspects of Plastics Recycling, ed. W. Hoyle and D. R. Karsa, Royal Society of Chemistry, Information Services, Cambridge, United Kingdom, 1997 Search PubMed.
- R. A. Gross and B. Kalra, Science, 2002, 297, 803–807 CrossRef CAS.
- R. A. A. Muzzarelli, in Chitin and Chitinases, ed. P. Jollès and R. A. A. Muzzarelli, Birkhäuser Verlag, Basel, Switzerland, 1999, pp. 1–6 Search PubMed.
- Dictionary of Renewable Resources, ed. H. Zoebelein, Wiley-VCH, Weinheim, Germany, 2001 Search PubMed.
- K. Kurita, Marine Biotechnol., 2006, 8, 203–226 Search PubMed.
- Metabolix, Inc. Announces Commencement of Construction of Commercial Manufacturing Facility for Biodegradable Natural Plastic, http://ir.metabolix.com/releasedetail.cfm%3FReleaseID%20%3D%20221691, accessed 8/14/2008 Search PubMed.
- M. N. Somleva, K. D. Snell, J. J. Beaulieu, O. P. Peoples, B. R. Garrison and N. A. Patterson, Plant Biotechnol. J., 2008, 6, 663–678 Search PubMed.
- Presidential Green Chemistry Challenge: 2002 Greener Reaction Conditions Award, http://www.epa.gov/gcc/pubs/pgcc/winners/grca02.html, accessed 5/21/2008 Search PubMed.
- M. G. Adsul, A. J. Varma and D. V. Gokhale, Green Chem., 2007, 9, 58–62 RSC.
- N. Ljungberg and B. Wesslén, Biomacromolecules, 2005, 6, 1789–1796 CrossRef CAS.
- V. L. Finkenstadt, C.-K. Liu, R. Evangelista, L. Liu, S. C. Cermak, M. Hojilla-Evangelista and J. L. Willett, Ind. Crops Prod., 2007, 26, 36–43 CrossRef CAS.
- V. L. Finkenstadt, L. Liu and J. L. Willett, J. Polym. Environ., 2007, 15, 1–6 CrossRef CAS.
- J. H. Clark, V. Budarin, F. E. I. Deswarte, J. J. E. Hardy, F. M. Kerton, A. J. Hunt, R. Luque, D. J. Macquarrie, K. Milkowski, A. Rodriguez, O. Samuel, S. J. Tavener, R. J. White and A. J. Wilson, Green Chem., 2006, 8, 853–860 RSC.
- G. W. Huber and A. Corma, Angew. Chem., Int. Ed., 2007, 46, 7184–7201 CrossRef CAS.
- K. Zweibel, J. Mason and V. Fthenakis, Sci. Am., 2008, 298, 64–73 Search PubMed.
- T. Colborn, D. Dumanoski and J. P. Myers, Our stolen future: are we threatening our fertility, intelligence, and survival? A scientific detective story, Dutton, New York, NY, 1996 Search PubMed.
- J. P. Sumpter and A. C. Johnson, Environ. Sci. Technol., 2005, 39, 4321–4332 CrossRef CAS.
- J. Thornton, Pandora's Poison: Chlorine, Health, and a New Environmental Strategy, MIT Press, Cambridge, MA, 2000 Search PubMed.
- C. Hogue, Chem. Eng. News, 2005, 83, 5.
- P. O. Darnerud, G. S. Eriksen, T. Johannesson, P. B. Larsen and M. Viluksela, Environ. Health Perspect. Suppl., 2001, 109, 49–68 CrossRef CAS.
- S. H. Swan, K. M. Main, F. Liu, S. L. Stewart, R. L. Kruse, A. M. Calafat, C. S. Mao, J. B. Redmon, C. L. Ternard, S. Sullivan, J. L. Teague, E. Z. Drobnis, B. S. Carter, D. Kelly, T. M. Simmons, C. Wang, L. Lumbreras, S. Villanueva, M. Diaz-Romero, M. B. Lomeli, E. Otero-Salazar, C. Hobel, B. Brock, C. Kwong, A. Muehlen, A. Sparks, A. Wolf, J. Whitham, M. Hatterman-Zogg and M. Maifield, Environ. Health Perspect., 2005, 113, 1056–1061 CAS.
- M. Bachran and T. Colborn, Summary and Comments on the TEDX Low-Dose BPA Spreadsheet, April 15, 2008.,http://www.endocrinedisruption.com/, accessed 8/13/2008 Search PubMed.
- M. A. Albrecht, C. W. Evans and C. L. Raston, Green Chem., 2006, 8, 417–432 RSC.
- N. D. Anastas and J. C. Warner, Chem. Health Saf., 2005, 12, 9–13 Search PubMed.
- R. L. Garrett, in Designing Safer Chemicals: Green Chemistry for Pollution Prevention, ed. S. C. DeVito and R. L. Garrett, American Chemical Society, Washington, DC, 1996, pp. 2–15 Search PubMed.
- S. C. DeVito, in Designing Safer Chemicals: Green Chemistry for Pollution Prevention, ed. S. C. DeVito and R. L. Garrett, American Chemical Society, Washington, DC, 1996, pp. 16–59 Search PubMed.
- R. S. Boethling, E. Sommer and D. DiFiore, Chem. Rev., 2007, 107, 2207–2227 CrossRef CAS.
- P. Galgali, A. J. Varma, U. S. Puntambekar and D. V. Gokhale, Chem. Commun., 2002, 2884–2885 RSC.
- P. T. Anastas, P. H. Bickart and M. M. Kirchhoff, Designing Safer Polymers, Wiley-Interscience, New York, NY, 2000 Search PubMed.
- W. L. Jorgensen, Science, 2004, 303, 1813–1818 CrossRef CAS.
- W. J. Welsh, W. Tong and P. G. Georgopoulos, in Computational Toxicology: Risk Assessment for Pharmaceutical and Environmental Chemicals, ed. S. Ekins, John Wiley & Sons, Inc., Hoboken, NJ, 2007, pp. 153–181 Search PubMed.
- C. Y. Wang, N. Ai, S. Arora, E. Erenrich, K. Nagarajan, R. Zauhar, D. Young and W. J. Welsh, Chem. Res. Toxicol., 2006, 19, 1595–1601 CrossRef CAS.
- L. Gunnarsson, A. Jauhiainen, E. Kristiansson, O. Nerman and D. G. J. Larsson, Environ. Sci. Technol., 2008, 42, 5807–5813 CrossRef CAS.
- D. B. Huggett, J. C. Cook, J. F. Ericson and R. T. Williams, Hum. Ecol. Risk Assess., 2003, 9, 1789–1799 CrossRef CAS.
- H. Feitsma and E. Cuppen, Mol. Cancer Res., 2008, 6, 685–694 CrossRef CAS.
- J. Berger and P. Currie, Curr. Med. Chem., 2007, 14, 2413–2420 CrossRef CAS.
- E. T. Gatzidou, A. N. Zira and S. E. Theocharis, J. Appl. Toxicol., 2007, 27, 302–309 CrossRef CAS.
- S. M. Reamon-Buettner, V. Mutschler and J. Borlak, Mutat. Res., 2008, 659, 158–165 CAS.
| This journal is © The Royal Society of Chemistry 2009 |