Ethanol fuel use in Brazil: air quality impacts
Larry G.
Anderson
*
Department of Chemistry, CB 194, University of Colorado Denver, POB 173364, Denver, Colorado 80217-3364, USA. E-mail: Larry.Anderson@ucdenver.edu; Fax: +1-303-556-4766; Tel: +1-303-556-2963
First published on 4th August 2009
Abstract
Brazil has a long history in the development of ethanol for use as a liquid fuel for vehicles. They have developed one of most efficient and economical systems for producing ethanol in the world. Brazil provides an example that many other countries would like to emulate. Using ethanol as a vehicle fuel has significant potential air quality impacts. This paper will review the available air quality and vehicle emissions data in Brazil, specifically focusing on vehicle related pollutants that may be impacted by the use of large quantities of ethanol in the fuel. The atmospheric concentrations of acetaldehyde (CH3CHO) and ethanol in Brazil are much higher than those in other areas of the world, while the concentrations of the single ring aromatic compounds and small carboxylic acids are more typical of observations elsewhere. Acetaldehyde and ethanol increase in vehicle emissions and nitrogen oxides (NOx) may increase when ethanol fuels are used. Both CH3CHO and NOx are very important contributors to photochemical air pollution and ozone (O3) formation. There are very significant O3 air quality problems in Brazil, most studied in the larger cities of São Paulo and Rio de Janeiro. These are issues that must be evaluated for other areas of the world that are considering the use of high ethanol content vehicle fuels.
Broader contextBrazil has a long history of using ethanol as a vehicle fuel, and has developed one of most efficient and economical systems for producing ethanol in the world. Brazil is an example that many other countries would like to emulate. Using large quantities of ethanol as a vehicle fuel has significant potential air quality impacts. This paper will review the available air quality and vehicle emissions data from Brazil, specifically focusing on vehicle related pollutants that may be impacted by the use of large quantities of ethanol in the fuel. Acetaldehyde and ethanol emissions increase in vehicles using ethanol fuels, and the atmospheric concentrations of these compounds are higher than most other areas of the world. Nitrogen oxides emissions may also increase when ethanol fuels are used. Both acetaldehyde and nitrogen oxides are important contributors to photochemical air pollution and ozone formation. There are very significant ozone problems in and around the larger cities of São Paulo and Rio de Janeiro, Brazil. This review is intended to highlight issues that must be considered and evaluated for other areas of the world that are considering the use of high ethanol content vehicle fuels. |
Introduction
Over the years, many articles have appeared in the popular press extolling the virtues of ethanol fuel use. Dickerson1 has published one such article. The article suggests that Brazil is on a path to independence from foreign oil, by developing its own oil reserves and by investing heavily in renewable energy from ethanol. About 40% of the fuel Brazilians use in their vehicles is ethanol (ton of oil equivalent (toe) basis). In addition, we are told that “environmentalists support the expansion of this clean, renewable fuel that has helped improve air quality in Brazil's cities.” What do we really know about air quality in Brazil? In this paper, we will review the available data on the air quality and vehicle emissions in the only area of the world (Brazil) that has used high ethanol content fuels for many years. The issues discussed should be considered by other countries that are considering the use of high ethanol content fuels.Niven2 has recently reviewed the environmental impacts and sustainability of ethanol in gasoline. Niven reviews data available on five environmental aspects of using ethanol blended with gasoline as a vehicle fuel. These include: (1) the effects on the emissions of air pollutants during the use of these fuels; (2) the impact of the fuels on subsurface soils and groundwater; (3) the effects of blending ethanol with the fuels on greenhouse gas emissions; (4) the energy efficiency of ethanol; and (5) the overall sustainability of ethanol production. Much of the discussion focuses on the use of 10% by volume blends of ethanol with gasoline (E10), the most commonly used blend globally. The conclusion of this work was that “E10 is of debatable air pollution merit (and may in fact increase the production of photochemical smog); offers little advantage in terms of greenhouse gas emissions, energy efficiency or environmental sustainability; and will significantly increase both the risk and severity of soil and groundwater contamination.”2 In order to supplant significant quantities of petroleum use as a vehicle fuel for light duty vehicles, high ethanol concentration blends such as 85% ethanol and 15% gasoline (E85) are the most likely alternative in the short term. Niven2 goes on to conclude that “E85 offers significant greenhouse gas benefits, however, it will produce significant air pollution impacts, involves substantial risks to biodiversity, and its groundwater contamination impacts and overall sustainability are largely unknown.” These latter conclusions for the high ethanol content fuels are based on very little data, because little data are available. It is generally believed that high ethanol content fuels will have less impact on groundwater than the fuels that they replace. The greenhouse gas benefits of high ethanol content fuels is also subject to considerable added uncertainty due to the impacts of land-use changes.3
Jacobson4 has constructed a model to evaluate the air quality and related health impacts of E85 fuel use in southern California and the US. Jacobson4 concludes that ozone (O3) would increase in southern California and the northeastern part of the US, which “may increase O3 related mortality, hospitalization, and asthma by about 9% in Los Angeles and 4% in the US as a whole, relative to 100% gasoline. E85 also increased peroxyacetyl nitrate (PAN) in the US but was estimated to cause little change in cancer risk.” Furthermore, “because of the uncertainty in future emission regulations, it can be concluded with confidence only that E85 is unlikely to improve air quality over future gasoline vehicles.”4 In addition, “unburned ethanol emissions from E85 may result in a global scale source of acetaldehyde larger than that of direct emissions.” The results of this work increased the controversy surrounding ethanol fuel use.
History of ethanol fuel use in Brazil
The use of ethanol as fuel for automobile engines in Brazil started in the 1920s. The mandatory use of 5% ethanol with gasoline as a blend was established by the Government in 1931 both because of the decline in international sugar prices and because all of the gasoline consumed in the country was imported.5 In 1933, the Sugar and Alcohol Institute (IAA) was created with the goal of expanding alcohol production capacity in order to deal with the overproduction of sugar.5 During World War II, the use of alcohol increased due to gasoline rationing. The use of alcohol declined in the 1950s and 1960s due to rising sugar prices, the decline in gasoline prices and PETROBRAS (Brazilian Petroleum Company) achieving self-sufficiency in oil refining.5In the early 1970s, Brazil's exports of sugar increased dramatically, with international sugar prices reaching a peak in November 1974.5 This was followed by a long and pronounced decline in sugar prices. At about the same time there was a sharp increase in oil prices which threatened the military dictatorship's ability to rule.6,7 At that time 90% of the gasoline was imported, causing fuel shortages, inflation, and diminished hard currency reserves. In November 1975, the National Alcohol Program (PNA), commonly known as “ProAlcool”, was set up to use domestic sources as a substitute for imported petroleum. The goal was to allow continued energy consumption and maintain economic growth.8 The program required use of 10% anhydrous ethanol as an additive to gasoline, with a voluntary component using 100% hydrated ethanol (95% ethanol + 5% water) in modified Otto cycle engines.7 During the first few years of the program, the objective was to install distilleries adjacent to existing sugar mills to produce anhydrous ethanol to be blended with gasoline.8
By 1979, the international oil crisis forced the Brazilian government to launch a second phase of the PNA. The goal was to use hydrous ethanol to completely substitute gasoline in automobiles.5 Agreement was reached between the government and automobile industry that allowed the program to proceed. With strong Government oversight, new distilleries were constructed for the large scale production of hydrous ethanol.8 This shift from anhydrous ethanol to hydrous ethanol production and the scale of the substitution had important repercussions on the balance of payments, on the automobile market, on oil refining and on the agricultural sector.5Fig. 1 shows new light duty vehicle production in Brazil as related to fuel used. This figure dramatically illustrates the growth in hydrous ethanol fueled vehicle production during the 1980s.
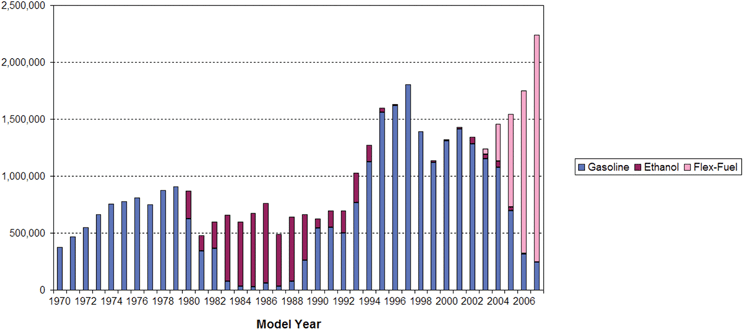 | ||
| Fig. 1 New light duty vehicle sales (non-diesel) by fuel type: gasoline (including gasohol), ethanol (hydrous) and flex fuel. Source: http://www.unica.com.br/downloads/estatisticas/eng/VEHICLES SALES IN BRAZIL.xls. | ||
Between 1975 and 1985, the production of sugarcane quadrupled and alcohol became a very important fuel used in the country.9 In 1984, the new civilian administration (the first after the military regime) cut public investment and subsidies.8 Due to the PNA program, about 94% of the new passenger vehicles were fueled by ethanol. By the late 1980s serious problems had developed. Large off-shore oil and natural gas reserves were discovered in the south and in the northeast of the country. There was a large drop in gasoline and fuel oil consumption in Brazil between 1973 and 1985 that was not easily accommodated by the refineries (see Fig. 2). As a result one-third of gasoline production, and one-fourth of fuel oil production, had to be exported at low prices. During 1989–1990 the surplus of gasoline, together with a combination of factors—economic recession; high inflation and alcohol producer debts; international debt crisis; the increase of international prices of sugar; the continued fall in international prices of petroleum; the lack of a coherent strategy for balancing the supply and demand for alcohol and gasoline; and the reduction in part of the subsidies to the program—national alcohol production changed from overproduction to a situation of deficit production.5 In 1990, a combination of bad climatic conditions together with a rise in the international price of sugar forced the Government to import alcohol (including methanol) for the first time to meet the national demand.5 Consumers had serious difficulties in getting alcohol to fill the tanks of their cars. In that year the production of alcohol-powered cars dropped from about 47% of new cars produced, to about 11%.
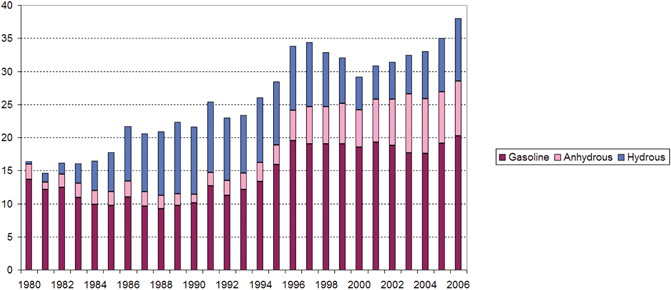 | ||
| Fig. 2 Annual production (in 106 m3 year−1) of gasoline, anhydrous ethanol and hydrous ethanol in Brazil. Source: ref. 8, http://www.unica.com.br/and and http://www.anp.gov.br/. | ||
In 1993, the government passed a law in which all gasoline marketed in Brazil would be blended with 20–25% of ethanol.6 In 1994 the Plan Real was launched with the objective of stabilizing the Brazilian economy and reducing inflation rates to single digits. The Federal Government policy was to force the sugar and ethanol industry to cut costs through higher productivity.8 In 1995 only 2.5% of all new cars were fueled by ethanol, but 35% (4.2 million) of the total number of cars were still powered with ethanol engines, the remaining 65% using a blend of ethanol with gasoline in amounts of about 22% ethanol.5 In 1995 Brazilian ethanol production again did not meet the national demand.5 In that year, 13.1 billion litres of alcohol were produced from 62% of the sugarcane crop and 1.8 billion litres of alcohol were imported, 40% as methanol and 60% as ethanol.
At the end of 1995, the Government officially launched the program, now called ‘new PNA’.5 Its new goal is to protect the environment, not to reduce oil dependence. Sugarcane prices, including freight to mills and distilleries, and all ethanol prices were deregulated and determined by market forces starting 1 January 1997.6 A special investment program was made available as an incentive for the continuation and the expansion of ethanol fuel production, to increase the research and production of new and improved engines, to reduce ethanol production costs, to increase the productivity of the ethanol industries and to reduce the amount of wastes.5
Currently the government's role in ethanol fuels is much less and much different than in earlier times. Its role is to ensure that the market-driven controls proceed smoothly, and to help improve the industry's environmental performance.6 Problems continue with the competition between the food and fuels sectors for sugarcane. In March 2006, the country's fuel blenders (e.g. BR (PETROBRAS), Shell, Exxon, Ipiranga) had to cut ethanol content to 20% of its blended fuel because of ethanol shortages.6 Sugar prices had risen to their highest levels in five years.
In March 2003, flexible (flex) fuel vehicles were introduced into the market in Brazil (see Fig. 1). These vehicles are designed to be fueled with gasohol, or ethanol, or any blend of gasohol and ethanol. For the customers, the value of the flex fuel vehicle is to allow the choice of fuel (each time the vehicle is refueled) according to characteristics of price, quality, performance and fuel availability. The vehicle uses special electronic sensors and an on-board computer to recognize the fuel composition and properly adjust the engine combustion parameters, without any interference from the driver. Fig. 1 shows the rapidly increasing importance of the flex fuel vehicle in Brazil's new car market.
Vehicle emissions
Vehicle emission standards
In the late 1980s, Brazil adopted regulations on vehicle emissions. The Vehicle Air Pollution Control Program (PROCONVE) established emission standards for new vehicles in Brazil. Table 1 shows the emissions limits set for new gasoline/ethanol fueled automobiles in Brazil.| Pollutanta | Phase I | Phase II | Phase III | Phase IV | Phase V |
|---|---|---|---|---|---|
| 1988 | 1992 | 1997 | 2007 | 2009 | |
| a CO—carbon monoxide; HC—hydrocarbon; NMHC—non-methane hydrocarbon; NOx—nitrogen oxides; PM—particulate matter. | |||||
| CO/g km−1 | 24.00 | 12.00 | 2.00 | 2.00 | 2.00 |
| HC/g km−1 | 2.10 | 1.20 | 0.30 | 0.30 | 0.30 |
| NMHC/g km−1 | — | — | — | 0.16 | 0.05 |
| NOx/g km−1 | 2.00 | 1.40 | 0.60 | 0.25 | 0.12 |
| PM/g km−1 | — | — | 0.05 | 0.05 | 0.05 |
| Aldehyde/g km−1 | — | 0.15 | 0.03 | 0.03 | 0.02 |
| Evaporative/g test−1 | 6.00 | 6.00 | 6.00 | 2.00 | 2.00 |
The addition of ethanol to gasoline increases the octane rating of gasoline, prevents pre-ignition, and can be used as a replacement for the tetraethyl lead additive used in gasoline.5 The widespread introduction of unleaded fuels throughout Brazil in 1975 allowed the level of lead in the air in urban areas to decrease by about 72% by the mid-1990s.13
Vehicle emission measurements
The vehicle emissions data described in this work are those which are available in the open literature. These data may not be representative of results that would be applicable to vehicles in widespread use.Onursal and Gautam12 have presented data for the average emissions of new light duty vehicles in the São Paulo metropolitan region. The vehicle emissions data show that the average emissions for the new vehicles are substantially below the emissions standards. The average carbon monoxide (CO) emissions for the gasohol fueled vehicles are higher than for the ethanol fueled vehicles. The hydrocarbon (HC) and nitrogen oxides (NOx) emissions are nearly the same for both fuel types, and the average aldehyde emissions are considerably higher for the ethanol fueled vehicles compared to gasohol. For the ethanol fueled vehicles, the HC emissions were about 70% ethanol, 10% aldehydes and 20% other organics, while for gasohol vehicles, the HC emissions were less than 10% each of ethanol and aldehydes, and more than 80% of the emissions were other organics. The aldehyde emissions from ethanol fueled vehicles were about 85% CH3CHO, 14% formaldehyde (CH2O) and 1% other aldehydes, while for gasohol vehicles CH2O was about 60% of the aldehyde emissions. These data have been updated and extended to model year 2007 vehicles, as shown in Table 2.14 Emissions data from flex fuel vehicles are included for the most recent years.
| Model year | Fuel type | Exhaust emissions/g km−1 | Evaporative/g test−1 | |||
|---|---|---|---|---|---|---|
| CO | HC | NOx | Aldehydes | |||
| a na—not available. | ||||||
| Pre-1980 | Gasoline | 54.0 | 4.7 | 1.2 | 0.050 | naa |
| 1980–1983 | Ethanol | 18.0 | 1.6 | 1.0 | 0.160 | naa |
| Gasohol | 33.0 | 3.0 | 1.4 | 0.050 | naa | |
| 1984–1985 | Ethanol | 16.9 | 1.6 | 1.2 | 0.180 | 10.0 |
| Gasohol | 28.0 | 2.4 | 1.6 | 0.050 | 23.0 | |
| 1986–1987 | Ethanol | 16.0 | 1.6 | 1.8 | 0.110 | 10.0 |
| Gasohol | 22.0 | 2.0 | 1.9 | 0.040 | 23.0 | |
| 1988–1989 | Ethanol | 13.1 | 1.6 | 1.3 | 0.110 | 10.0 |
| Gasohol | 16.9 | 1.6 | 1.7 | 0.040 | 23.0 | |
| 1990–1991 | Ethanol | 9.6 | 1.2 | 1.1 | 0.110 | 1.8 |
| Gasohol | 12.4 | 1.4 | 1.4 | 0.040 | 2.7 | |
| 1992–1993 | Ethanol | 3.9 | 0.6 | 0.6 | 0.038 | 1.0 |
| Gasohol | 6.2 | 0.6 | 0.7 | 0.018 | 1.9 | |
| 1994–1995 | Ethanol | 4.6 | 0.7 | 0.7 | 0.042 | 0.9 |
| Gasohol | 5.4 | 0.6 | 0.6 | 0.030 | 1.6 | |
| 1996–1997 | Ethanol | 2.4 | 0.5 | 0.5 | 0.026 | 1.0 |
| Gasohol | 2.5 | 0.3 | 0.4 | 0.013 | 1.1 | |
| 1998–1999 | Ethanol | 0.63 | 0.18 | 0.23 | 0.014 | 1.48 |
| Gasohol | 0.77 | 0.17 | 0.27 | 0.006 | 0.80 | |
| 2000–2001 | Ethanol | 0.64 | 0.16 | 0.15 | 0.015 | 1.33 |
| Gasohol | 0.60 | 0.12 | 0.17 | 0.004 | 0.70 | |
| 2002–2003 | Ethanol | 0.75 | 0.16 | 0.09 | 0.018 | naa |
| Gasohol | 0.41 | 0.11 | 0.12 | 0.004 | 0.68 | |
| 2004–2005 | Ethanol | 0.82 | 0.17 | 0.08 | 0.016 | naa |
| Gasohol | 0.35 | 0.10 | 0.07 | 0.004 | 0.69 | |
| Flex-eth | 0.42 | 0.14 | 0.12 | 0.014 | naa | |
| Flex-gas | 0.42 | 0.10 | 0.05 | 0.003 | naa | |
| 2006 | Ethanol | 0.67 | 0.12 | 0.05 | 0.14 | naa |
| 2006–2007 | Gasohol | 0.33 | 0.08 | 0.08 | 0.002 | 0.46 |
| Flex-eth | 0.47 | 0.11 | 0.07 | 0.014 | 1.27 | |
| Flex-gas | 0.48 | 0.10 | 0.05 | 0.003 | 0.62 | |
Emissions measurements have been made on two different three-way catalyst equipped cars (1980 GM Opala and 1982 VW Gol) operating on hydrated ethanol.15,16 Acetaldehyde is the major carbonyl emission from these vehicles, contributing 88–99% of the sum of CH2O and CH3CHO emitted during different phases of the emissions tests for the two vehicles. The mass emissions for the GM Opala were 0.046 g km−1 and 0.246 g km−1 for CH2O and CH3CHO without a catalyst, and 0.014 g km−1 and 0.044 g km−1 with the catalyst installed. The emissions from the VW Gol were 0.019 g km−1 and 0.143 g km−1 for CH2O and CH3CHO without a catalyst, and 0.005 g km−1 and 0.023 g km−1 with the catalyst installed. On a molar basis the CH2O/CH3CHO ratio in emissions were 0.26 and 0.19 without the catalyst, and 0.45 and 0.30 with the catalyst.17
Coutrim et al.18 have reported emissions from two test engines without catalysts for aldehydes from ethanol blended fuels. One test engine was fueled with an ethanol rich fuel (82% hydrous ethanol with 18% gasoline) and the second with gasohol (22% anhydrous ethanol with 78% gasoline). The higher ethanol fueled engine produced about 1.7 times as much CH2O and 3.5 times as much CH3CHO as did the gasohol engine. The molar CH2O/CH3CHO ratio for these two fuels was 0.42 for the ethanol rich fuel, and 0.86 for the gasohol fuel.
Correa et al.19 performed emissions tests on three ethanol fueled vehicles (1992, 1994, and 1995 model years) and three gasohol fueled vehicles (1996, 1997, and 2000 model years) running at a constant 800 rpm. The ethanol fueled vehicles are older which is typical of this portion of the vehicle fleet. The molar CH2O/CH3CHO ratio in emissions from the ethanol fueled vehicles was 0.68, and 0.75 for the gasohol fueled vehicles. In these tests, the emissions of aldehydes were lower for the older ethanol fueled vehicles than for the newer gasohol fueled vehicles.
Sales and Sodre20 modified the cold start system for an ethanol fueled vehicle in an attempt to reduce exhaust emissions for the vehicle. It was found the HC and CO emissions could be reduced by up to 8.6 and 17.2% and allowed a faster start with the implementation of changes in the cold start system. Essentially no change in NOx and aldehyde emissions was found as a result of these tests. The average emissions from this 1.0 L four-cylinder engine-powered vehicle operated on the FTP-75 test cycle ranged from 1.68 to 1.84 g km−1 for HC, from 7.29 to 8.80 g km−1 for CO, from 0.34 to 0.35 g km−1 for aldehydes, and from 1.32 to 1.36 g km−1 for NOx for the four test configurations studied in this work.
Lucon et al.21 have presented CETESB (the state environmental agency in São Paulo) emissions data for model year 2003 and 2004 new vehicles. These vehicles consisted of three types: conventional vehicles designed for gasohol use; conventional vehicles designed for ethanol use; and flex fuel vehicles. The flex fuel vehicles were tested on both gasohol and ethanol. Since conventional vehicles that operate on ethanol fuels are such a small part of the new vehicle market, comparisons of emissions of flex fuel vehicles with the two different fuels with conventional gasohol vehicles will be discussed. The emissions of HC, NOx and total aldehydes were lower for flex fuel vehicles operating on gasohol than conventional gasohol vehicles by 41%, 56% and 12% respectively, but CO emissions were higher by 18%. For the flex fuel vehicles operating on ethanol compared to conventional gasohol vehicles, the emissions of CO, HC and NOx increased by 30–36%, while the total aldehyde emissions increased by an average of over 300%. The average emissions for all of these new vehicles are lower than the emissions standards for these vehicles. The fuel economy of flex fuel vehicles operating on gasohol was about 6% lower, and operating on ethanol was about 37% lower, than for conventional vehicles operating on gasohol.
de Abrantes et al.22 have reported results of the measurements of emissions of polycyclic aromatic hydrocarbons (PAHs) from vehicles fueled with gasohol and with ethanol. The average emissions of PAHs from gasohol fuel use were higher than those with ethanol fuel use, averaging 0.832 µg TEQ km−1 (BAP eq) (TEQ—toxicity equivalences; BAP eq—benzo(a)pyrene equivalences) for the gasohol tests and 0.016 µg TEQ km−1 (BAP eq) for the ethanol tests. Fuel additives and oil composition had a significant effect on PAH emissions with the gasohol fuel, but no significant effect with ethanol fuel.
Diesel vehicles contribute significantly to the total vehicle emissions and hence to air quality in Brazil. de Abrantes et al.23,24 present results of standard dynamometer FTP-75 emissions tests on four Brazilian light duty diesel vehicles. The average CH3CHO emission for two tests on all four vehicles was 15.5 mg km−1 and the average CH2O emission was 43.2 mg km−1. For these diesel vehicle emissions tests the average molar CH2O/CH3CHO ratio was 3.9. All of the vehicles tested met the vehicle emissions standards for the years they were produced, but none met the 2009 aldehyde emission standard of 20 mg km−1. The CH2O/CH3CHO emissions ratios from diesel vehicles in Brazil are much higher that the corresponding ratios for gasohol and ethanol fueled vehicles. Molar ratios of CH2O/CH3CHO are used throughout this paper to aid in identifying the source of these compounds, from emissions and in the ambient air.
Correa and Arbilla have reported results of aromatic HC25 and carbonyl26 emissions with diesel and biodiesel fuels from a heavy duty diesel engine typical of that used in buses in Brazil. The fuels tested were commercial diesel, and 2%, 5%, and 20% (B2, B5, B20) biodiesel blends with diesel. The biodiesel was made by esterification of castor oil with ethanol. The use of the biodiesel blends led to significant reductions in the emissions of the monocyclic aromatic HCs, including the BTEX compounds (benzene, toluene, ethylbenzene and xylenes), by about 5%, 9% and 22% relative to the diesel fuel. Similar reductions in PAH emissions were found with increasing concentration of biodiesel. The tests for effects on carbonyl emissions included a 10% (B10) biodiesel blend in addition to the fuels identified above.25 The emissions of the aliphatic carbonyl compounds increased systematically with increasing biodiesel in the blends. Guarieiro et al.27 have found different results from their study of carbonyl emissions from diesel and biodiesel blends. The fuels studied ranged from pure diesel to pure biodiesel. The data show no significant trend in the CH2O emissions as fuel composition changed, but CH3CHO emissions decreased slightly with increasing biodiesel. The sampling techniques for carbonyl compounds used in this study were different than the previous study and these tests were conducted on a small two-cylinder diesel engine. In addition, the biodiesels used were different—a methyl soy biodiesel and a methyl residual oil biodiesel.
Merritt et al.28 have reported emissions measurements from ethanol–diesel blends. This may become an important petroleum substitute in Brazil, but there are some technical barriers that must be overcome prior to its use. The test data were collected from three non-road diesel engines using a base diesel fuel, and diesel fuel blended with 7.7%, 10% and 15% ethanol by volume. Formaldehyde emissions for the three ethanol blended fuels increased slightly by an average of 3.6%, 6.7% and 22%, while CH3CHO emissions increased by an average of 41%, 57% and 104% respectively. The only other emissions to change significantly were those of ethanol which was the largest of the measured emissions, and it increased in proportion to the quantity of ethanol blended with the fuel. The emissions of benzene decreased slightly with increasing ethanol, but other aromatic HC emissions varied both up and down.
Compressed natural gas (CNG) is a fuel that is being used in increasing quantities as a vehicle fuel in Brazil. Many CNG vehicles are dual fueled vehicles which can use CNG or gasohol. Correa and Arbilla29 performed emissions tests on 20 of these dual fueled vehicles operating at a constant 2500 rpm. When natural gas was used as the fuel, the average CH2O/CH3CHO ratio was 3.42. When these same vehicles were run on gasohol fuel, the average CH2O/CH3CHO ratio was 0.24. Consistently, the CNG fueled test emitted more CH2O and less CH3CHO than the gasohol fuel test.
Martins et al.30 have reported the results of extensive measurements of CO, CO2, NOx, sulfur dioxide (SO2) and individual HC and carbonyl compounds both inside and outside of tunnels in São Paulo, Brazil. Samples were collected in the Janio Quadros Tunnel during late March 2004 and in the Maria Maluf Tunnel in early May 2004. On average 95% of the vehicles passing through the Janio Quadros Tunnel use either gasohol or hydrous ethanol, with the remaining vehicles being light duty vehicles burning diesel fuel. On average 13% of the vehicles using the Maria Maluf Tunnel are heavy duty diesel fueled, with the remaining fueled by gasohol or hydrous ethanol. These data were used to determine emission factors for various compounds from the vehicle fleet. For the light duty vehicles the emissions factors for CO and NOx were found to be 14.6 ± 2.3 g km−1 and 1.6 ± 0.3 g km−1 respectively, while for the heavy duty vehicles these emissions factors were found to be 20.6 ± 4.7 g km−1 and 22.3 ± 9.8 g km−1 respectively. The average emission factors for the following organic compounds in the Maria Maluf Tunnel were: CH2O 48.4 ± 35.1 mg km−1; CH3CHO 45.7 ± 29.1 mg km−1; benzene 78.3 ± 72.0 mg km−1; toluene 134.5 ± 135.4 mg km−1; ethylbenzene 31.1 ± 33.0 mg km−1; m,p-xylene 62.0 ± 62.8 mg km−1; and o-xylene 44.4 ± 39.8 mg km−1. The average molar CH2O/CH3CHO ratio in emissions from these vehicles is 1.5.
Teixeira et al.31 have done an evaluation of mobile source emissions for the metropolitan area of Porto Alegre. This work shows that gasohol fueled vehicles contribute the largest portions of CO and HC emissions, while diesel vehicles contribute the largest portions of NOx, sulfur oxides (SOx) and particulate emissions. Gasohol and ethanol fueled vehicles are the major contributors to aldehyde emissions. This study generated total annual vehicle emissions for these pollutants from the early 1980s through to 2004. The emission of CO, NOx, HC and particulates showed a significant growth through the early 1990s, followed by a gradual decline from the mid-1990s through to 2004. It was also shown that aldehyde emissions increased gradually through the entire period. The average emissions for ethanol fueled vehicles in 2004 exceeded the emissions standards for CO and HC, while the average emissions of diesel vehicles exceeded the emissions standards for NOx.
The CETESB32 has presented average emissions data for the fleet of vehicles operating on gasohol and ethanol in the São Paulo metropolitan region. For the fleet of gasohol fueled vehicles the average CO emissions decreased from about 17 g km−1 in 1997 to about 11 g km−1 in 2006, the average HC emissions decreased from about 1.5 g km−1 in 1997 to about 1.1 g km−1 in 2006, and the average NOx emissions decreased from about 0.9 g km−1 in 1997 to about 0.7 g km−1 in 2006. During this same time period for the fleet of ethanol fueled vehicles the average CO emissions increased from about 16 g km−1 to 20 g km−1 by 2006, the average HC emissions increased from about 1.9 g km−1 to 2.1 g km−1 by 2006, and the average NOx emissions increased from about 1.1 and 1.3 g km−1 between 1997 and 2006. These data show that the fleet average emission rates for CO, HCs and NOx for the ethanol fueled vehicles are higher than those from the gasohol fueled fleet. As can be seen from Fig. 1, very few new ethanol fueled vehicles have been sold since 1997, hence this portion of the fleet is getting older and many of the old ethanol fueled vehicles are still in the fleet. The gasohol fueled portion of the fleet has continued to grow with the addition of new gasohol-powered vehicles. Domingues and Gatti33 have reported some speciated volatile organic compound (VOC) data for emissions from gasohol vehicles, analyzing 75 individual VOC compounds. They found that the top ten VOC compounds in emissions (excluding the carbonyl compounds) were: toluene; p-xylene; 2-methylbutane; 2,2,3,3-tetramethyl-butane; benzene; pentane; ethane; 1,3-butadiene; m-xylene and o-xylene.
A recent emission inventory (2005) for the city of São Paulo concluded that CH3CHO and CH2O account for 3% and 4% respectively of the VOC emissions.25 The recent increases in the emissions of these compounds may be due to the use of ethanol, gasohol and natural gas fuels, but the increased use of biodiesel may also be contributing to these emissions changes.19,26,29
Air quality effects
Romieu et al.34 discuss some of the early air quality impacts of increased use of ethanol fuels in São Paulo, Brazil. About 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cars used ethanol fuel in 1981 which increased to about 500
000 cars used ethanol fuel in 1981 which increased to about 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by 1985. At the same time there was an 18.2% decrease in the number of vehicles using gasoline. The main impacts on air quality indices due to the shift in fuel consumption were: decreased SO2; maximum CO values tended to be lower, but still exceeded standards at all monitoring locations; lead in the air decreased from 1 µg m−3 in 1978 to 0.3 µg m−3 in 1983.34 More recently, the annual average concentrations of lead in São Paulo ranged from a high of 0.17 µg m−3 to a low of 0.06 µg m−3 at the four sites monitored during 1997 and 2003.14 It was suggested that O3 exposure may not be properly assessed in the early years due to the small number of monitoring stations in the area. The high levels of HCs, the levels of nitrogen dioxide (NO2) and geographical factors suggest high production of oxidants. Romieu et al.34 suggested that the shift towards increased ethanol fuel use increases the emissions of aldehydes to the air which could lead to an increase in O3 production.
000 by 1985. At the same time there was an 18.2% decrease in the number of vehicles using gasoline. The main impacts on air quality indices due to the shift in fuel consumption were: decreased SO2; maximum CO values tended to be lower, but still exceeded standards at all monitoring locations; lead in the air decreased from 1 µg m−3 in 1978 to 0.3 µg m−3 in 1983.34 More recently, the annual average concentrations of lead in São Paulo ranged from a high of 0.17 µg m−3 to a low of 0.06 µg m−3 at the four sites monitored during 1997 and 2003.14 It was suggested that O3 exposure may not be properly assessed in the early years due to the small number of monitoring stations in the area. The high levels of HCs, the levels of nitrogen dioxide (NO2) and geographical factors suggest high production of oxidants. Romieu et al.34 suggested that the shift towards increased ethanol fuel use increases the emissions of aldehydes to the air which could lead to an increase in O3 production.
Gurjar et al.35 have evaluated emissions and air quality in 18 mega-cities, based on World Bank and United Nations data. They have used a multi-pollutant index based on three criteria pollutants: total suspended particulates (TSP), SO2, and NO2. Two of the mega-cities in this evaluation are in Brazil—São Paulo and Rio de Janeiro. Based on this multi-pollutant index, Rio de Janeiro ranks as the 12th most polluted and São Paulo ranks as the 17th most polluted. None of the pollutants in this index are part of the specific discussions of air quality impacts of ethanol fuel use.
In this paper, atmospheric measurements will be reported that are available in the open literature. These measurements may include very small data sets, widely varying sampling locations, sampling periods and seasonality, and differing sampling and analysis techniques. These differences will make it difficult to compare results from these studies.
Atmospheric aldehydes
The results of early measurements of total aldehyde concentrations in São Paulo have been reported.36 These measurements of total aldehydes were made using the 3-methyl-2-benzothiazolinone hydrochloride (MBTH) method. This method measures total aldehydes, not individual compounds. The concentration results are presented as CH2O. Long-term monitoring was reported for four sites in São Paulo. This long-term monitoring consisted of 24 h sampling periods on weekdays (Monday–Friday) at each of the four sites from July 1980 to June 1981. The average concentrations of the total aldehydes reported at each of these sites over the year are shown in Table 3. The Praça do Correio and Congonhas sites are believed to be more heavily influenced by primary aldehydes, directly emitted from motor vehicles, since the mean CO concentrations were highest at these two sites. These concentrations are quite high compared to measurements in other areas of the world. Table 3 summarizes the measurements of CH2O and CH3CHO made in the São Paulo area.| Month/year | Location | CH2O/ppb | CH3CHO/ppb | CH2O/CH3CHO ratio | Number of samples | Sample period/h | Ref.a |
|---|---|---|---|---|---|---|---|
| a Thesis and CETESB refer to a thesis or CETESB report cited in the numbered reference. | |||||||
| Jul. 1980–Jun. 1981 | Praça do Correio | Total aldehydes | 42 | — | ∼260 | 24 | 36 |
| Jul. 1980–Jun. 1981 | Parque Dom Pedro | Total aldehydes | 20 | — | ∼260 | 24 | 36 |
| Jul. 1980–Jun. 1981 | Mooca | Total aldehydes | 15 | — | ∼260 | 24 | 36 |
| Jul. 1980–Jun. 1981 | Congonhas | Total aldehydes | 27 | — | ∼260 | 24 | 36 |
| Oct. 1986 | Praça do Correio | 5.4 | 16.1 | 0.3 | 3 | 2 | 39 |
| Jun.–Jul. 1988 | Univ. São Paulo | 8.8 | 7.6 | 2.5 | 8 | 2–4 | 39 |
| Jul. 1988 | CETESB | 13.5 | 8.1 | 2.2 | 4 | 1.5 | 39 |
| Dec. 1988 | Congonhas | 14.5 | 24.2 | 0.6 | 26 | — | Thesis-50 |
| 1988 | — | 16.3 | 30.6 | 0.5 | 47 | — | Thesis-61 |
| Jul. 1989 | — | 4.5 | 10.5 | 0.4 | 32 | — | Thesis-61 |
| Sep.–Oct. 1989 | Congonhas | 10.8 | 22.3 | 0.5 | 17 | — | CETESB-50 |
| Mar.–Apr. 1990 | Cerqueira César | 15.5 | 24.3 | 0.6 | 23 | — | CETESB-50 |
| Apr. 1990 | Mooca | 8.5 | 16.2 | 0.5 | 12 | — | CETESB-50 |
| Aug. 1990 | Cerqueira César | 21.8 | 27.3 | 0.8 | 6 | — | CETESB-50 |
| Jan. 1993 | Six locations | 7.8 | 11.3 | 0.7 | 6 | 3–8 | 40 |
| 1993 | Mooca | 4.2 | 6.1 | 0.7 | 179 | — | CETESB-50 |
| 1993 | Cerqueira César | 7.6 | 10.6 | 0.7 | 180 | — | CETESB-50 |
| Oct. 96–Jan. 97 | Cerqueira César | 5.4 | 7.5 | 0.7 | 132 | — | CETESB-50 |
| Oct. 96–Jan. 97 | Univ. São Paulo | 1.3 | 2.8 | 0.5 | 60 | — | CETESB-50 |
| Jan.–Jul. 1997 | Several sites | 9 | 10 | — | 39 | 3 | 46 |
| Jul.–Sep. 1997 | Cerqueira César | 7.0 | 11.7 | 0.6 | 155 | — | CETESB-50 |
| Jul.–Sep. 1997 | Univ. São Paulo | 4.2 | 9.2 | 0.5 | 130 | — | CETESB-50 |
| Feb. 1998 | Univ. São Paulo | 5 | 5.4 | 0.9 | 11 | 1 | 47 |
| Aug. 1999 | Água Funda | 12.3 | 11.3 | 1.3 | 32 | 2 | 49 |
| Aug. 1999 | Cidade Universitária | 13.9 | 12.5 | 1.2 | 34 | 2 | 49 |
| Nov. 2000 | Univ. São Paulo | 4.2 | — | — | 18 | 2 | 51 |
| Aug. 2001 | Janio Quadros Tunnel | 24–31 | 34–35 | 0.9 | — | 2 | 52 |
| Aug. 2001 | Ambient-Janio Quadros | 3.3–46 | 1.2–57 | 1.4 | — | 2 | 52 |
| Oct. 2001 | Maria Maluf Tunnel | 28–39 | 25–32 | 1.0 | — | 2 | 52 |
| Oct. 2001 | Ambient-Maria Maluf | 1.0–46 | 4.2–51 | 1.4 | — | 2 | 52 |
| Aug. 2002 | Univ. São Paulo | 4.0 | — | — | 30 | 1 | 51 |
| Mar.–Apr. 2004 | Fourteen locations | 18.1 | 15.4 | 1.2 | 14 | 1 | 59 |
| Jul.–Oct. 2006 | Cerqueira César | 5.7 | 5.6 | 1.0 | 141 | 2 | 32 |
| Feb. 2007 | Univ. São Paulo | 13.5 | — | — | 5 | 2 | 51 |
Tanner et al.37 have reported results of CH2O and CH3CHO measurements made using the 2,4-dinitrophenylhydrazine (DNPH) coated cartridge technique. Twelve-hour samples were collected for a four day period (1–5 July, 1985) at the Vila Isabel site in Rio de Janeiro, Brazil. In addition, 1- or 2-h samples were collected at this site on 2 and 4 July, and at the Pontifical Catholic University of Rio de Janeiro (PUC/RJ) site on 9 July. The CH2O and CH3CHO concentrations for Rio de Janeiro are shown in Table 4. For the 12 h data, the nighttime average ratio of CH2O/CH3CHO = 0.57 ± 0.12, which was lower than the daytime ratio of 0.80 ± 0.07. This nighttime ratio agrees well with the ratio of CH2O/CH3CHO = 0.60 ± 0.02 measured in the Santa Barbara Tunnel in Rio de Janeiro during April 1985. The average of the 1 h and 2 h daytime aldehyde concentration measurements made at the Vila Isabel site were much higher than the daytime average concentration measurements made at the PUC/RJ site. The measurements at this site were influenced by a strong sea-breeze, and were much lower than data collected at the downtown site, but still well above the marine background levels reported by Lowe and Schmidt38 in the south Atlantic. These results suggest that aldehyde concentrations, particularly CH3CHO, are increased considerably by additional primary emission sources in Rio de Janeiro that are not common in other countries. These sources are likely to be ethanol fueled and gasohol fueled motor vehicles.
| Month/year | Location | CH2O/ppb | CH3CHO/ppb | CH2O/CH3CHO ratio | Number of samples | Sample period/h | Ref.a |
|---|---|---|---|---|---|---|---|
| a Thesis and Conf. refer to a thesis or conference report cited in the numbered reference. b Period when the sample was collected is not stated in the reference. | |||||||
| Apr. 1985 | Santa Barbara Tunnel | 10.6 | 17.1 | 0.6 | 8 | — | 37 |
| Jul. 1985 | Villa Isabel | 9.8 | 13.5 | 0.7 | 7 | 12 | 37 |
| Jul. 1985 | Villa Isabel | 26 | 37 | 0.7 | 15 | 1–3 | 37 |
| Jul. 1985 | Gávea (PUC) | 1.8 | 3.7 | 0.5 | 10 | 1–2 | 37 |
| Jan. 1987 | Gávea | 4.6 | 6.2 | 0.8 | 2 | — | Thesis-50 |
| Jan. 1987 | Santa Barbara Tunnel | 65 | 236 | 0.3 | 2 | 1 | 39 |
| Jan. 1987 | Rebouças Tunnel | 154 | 307 | 0.5 | 1 | — | Thesis-61 |
| Sep. 1987 | R. Cosme Velho | 4.1 | 4.7 | 0.9 | 6 | 2–7 | 39 |
| Jan. 1993 | Six locations | 9.7 | 28 | 0.5 | 6 | 6–8 | 40 |
| Dec. 1995 | One location | 11.8 | 9.9 | 1.2 | 11 | 6 | 42 |
| 1997??b | Prédio Centro | 5.8 | 6.3 | — | — | — | Conf.-43 |
| 1997??b | FIOCRUZ | 2.7 | 9.3 | — | — | — | Conf.-43 |
| Dec. 1998 | Av. Presidente Vargas | 19.5 | 21.2 | 1.0 | 4 | 2 | 19 |
| 1998??b | FIOCRUZ | 4.0 | 2.7 | — | — | — | Conf.-43 |
| Aug. 1999 | Av. Presidente Vargas | 3.4 | 4.9 | 0.7 | 15 | 2 | 19 |
| May–Nov. 2000 | Av. Presidente Vargas | 8.8 | 5.8 | 1.5 | 13 | 2–3 | 50 |
| Aug.–Nov. 2000 | Av. Presidente Vargas | 16.8 | 15.3 | 1.2 | 6 | 2–3 | 19 |
| Jan. 2001 | Av. Presidente Vargas | 51 | 41 | 1.2 | 3 | 2 | 19 |
| Aug.–Dec. 2001 | Av. Presidente Vargas | 52 | 38 | 1.4 | 6 | 3 | 29 |
| Feb.–Dec. 2002 | Av. Presidente Vargas | 68 | 15.8 | 4.7 | 18 | 1.5–3 | 29 |
| Apr.–Nov. 2002 | FIOCRUZ | 132 | 35 | 4.0 | 45 | 2 | 54 |
| Apr. 2002–Feb. 2003 | Tijuca District | 151 | 30 | 4.9 | 86 | 2 | 55 |
| Dec. 2003 | Eight locations | 10.1 | 11.1 | 0.9 | 8 | 1 | 58 |
| Nov. 2005–Aug. 2006 | Bus, air-conditioned | 39 | 32 | 1.6 | 6 | — | 60 |
| Nov. 2005–Aug. 2006 | Bus station | 118 | 89 | 1.4 | 5 | — | 60 |
| 2008 | Praça Saens Peña | 53.0 | 16.9 | 3.1 | 28 | 2 | 63 |
| 2008 | Entrada Floresta da Tijuca | 22.7 | 6.9 | 3.3 | 40 | 2 | 63 |
| 2008 | Interior Floresta da Tijuca | 9.4 | 3.1 | 3.0 | 40 | 2 | 63 |
| 2008 | Alto Floresta da Tijuca | 13.1 | 4.5 | 2.9 | 34 | 2 | 63 |
Grosjean et al.39 present results of carbonyl sampling studies during the late 1980s using DNPH coated cartridges conducted at three sites in São Paulo, two sites in Rio de Janeiro, and one site in Salvador. The results of these measurements made in the different areas of Brazil are presented in Table 3 for São Paulo, Table 4 for Rio de Janeiro and Table 5 for Salvador. The sampling was done during the winter and early spring to minimize the contribution of photochemistry, and one of the sites in Rio de Janeiro was in a tunnel which would be more representative of on-road vehicle emissions. It was concluded39 that CH2O concentrations measured in Brazil are as high as those measured elsewhere in the world, but not substantially higher, while CH3CHO concentrations were substantially higher, and the CH2O/CH3CHO ratio was consistently lower than that measured elsewhere. Furthermore, the tunnel data clearly show very high levels of vehicle emissions of CH3CHO compared to CH2O. The very high ambient concentrations of CH3CHO are likely due to the large scale use of ethanol as a vehicle fuel.
| Month/year | Location | CH2O/ppb | CH3CHO/ppb | CH2O/CH3CHO ratio | Number of samples | Sample period/h | Ref. |
|---|---|---|---|---|---|---|---|
| a Period when the sample was collected is not stated in the reference. | |||||||
| Salvador | |||||||
| Sep. 1988 | Vitoria street | 8.9 | 19 | 0.5 | 3 | 1 | 39 |
| 1993??a | Bus station | 18.5 | 5.8 | 4.5 | 12 | 1 | 41 |
| Feb. 1992 | Tunnel | 80 | 75 | 1.1 | 4 | 1 | 41 |
| Jan. 1992 | Bus station | 52 | 12 | 4.4 | 11 | 1–2 | 44 |
| 1995??a | Mall park-enclosed | 8.3 | 13 | 0.7 | 5 | 1–2 | 44 |
| 1995??a | Business park-enclosed | 44 | 69 | 0.7 | 7 | 1–2 | 44 |
| 1995??a | Tunnel | 80 | 72 | 1.2 | 4 | 1–2 | 44 |
| Apr. 1992 | Rio Vermelho | 2.9 | 3.5 | 1.0 | 17 | 1–2 | 44 |
| Dec. 1991 | Baixa dos Sapateiros | 11 | 6.3 | 1.8 | 24 | 1–2 | 44 |
| 1995??a | Lagoa Verde | 1.5 | 2.1 | 0.8 | 17 | 1–2 | 44 |
| 1995??a | Cacha-Pregos | 1.2 | 1.2 | 1.1 | 20 | 1–2 | 44 |
| Porto Alegre | |||||||
| Apr. 1997 | Rodoviaria | 15.7 | 17.7 | 0.9 | 6 | 1 | 45 |
| Apr. 1997–Apr. 1999 | Rodoviaria-morning | 7.2 | 3.5 | 2.26 | 111 | 3 | 48 |
| Apr. 1997–Apr. 1999 | Rodoviaria-evening | 5.0 | 3.0 | 1.75 | 22 | 3 | 48 |
| May–Sep. 1999 | Rodoviaria-morning | 4.6 | 3.85 | 1.25 | 24 | 3 | 48 |
| May–Sep. 1999 | Rodoviaria-evening | 4.8 | 4.7 | 1.07 | 21 | 3 | 48 |
| Fortaleza | |||||||
| 2003 | Federal Univ. of Ceará | 18.2 | 15.4 | 1.2 | 2 | 56 | |
| Nov.–Dec. 2004 | Federal Univ. of Ceará | 2.3 | 0.4 | 4 | 12 | 1.5–2 | 57 |
| Londrina | |||||||
| Jan.–Feb. 2002 | Bus station | 7.9 | 1.3 | 6.3 | 14 | 24 | 53 |
| Jan.–Feb. 2002 | Central | 4.1 | 3.0 | 1.4 | 16 | 24 | 53 |
| Jul. 2002 | Central | 5.1 | 5.7 | 0.9 | 15 | 24 | 53 |
| Jul. 2002 | Agricultural | 1.0 | 0.4 | 2.5 | 14 | 24 | 53 |
Several authors have reported measurements of CH2O and CH3CHO made both in indoor and outdoor air.40,42,46,56,57,60 The outdoor data collected in these studies and measurements made in bus stations and parking garages are included in the data tables (Tables 3, 4 and 5).
de Andrade et al.41 have reported the measurements of both gas phase and aerosol phase CH2O and CH3CHO in two source rich areas of Salvador, Brazil: (1) a bus station where the predominant traffic is diesel fueled buses; and (2) a tunnel where about 41% of the traffic is fueled by hydrous ethanol, about 50% by gasohol (approx. 22% ethanol) and about 8% by diesel fuel. The gas phase CH2O and CH3CHO concentrations measured in the bus station averaged 18.5 ppb and 5.8 ppb, while the aerosol concentrations averaged 0.014 µg m−3 and 0.038 µg m−3 for n = 12 samples. The gas phase CH2O and CH3CHO concentrations measured in the tunnel averaged 79.5 ppb and 75.0 ppb, while the aerosol concentrations averaged 0.163 µg m−3 and 0.060 µg m−3 for n = 4 samples. This work confirmed that more than 99% of the CH2O and CH3CHO are present in the gas phase. The gas phase CH2O/CH3CHO ratio for the bus station data was 4.5 ± 3.0 and for the tunnel was 1.1 ± 0.2 (Table 5). The high CH2O/CH3CHO ratio in the bus station is due to the much higher emissions of CH2O from the diesel fueled buses.
de Andrade et al.44 have reported results for measurements of CH2O and CH3CHO at six sites in Salvador, a fishing village and rural area in the vicinity of Salvador. There is no indication in the paper when these measurements were made. Four of the sites were enclosed. One of these was a bus station which served diesel fueled buses. The average concentration for CH2O was 52 ppb, for CH3CHO was 12 ppb, and the average CH2O/CH3CHO ratio was 4.4. Sampling was done in an enclosed mall parking lot and an enclosed business parking lot. For the mall lot, the average concentration of CH2O was 8.3 ppb, that for CH3CHO was 13 ppb, and the average CH2O/CH3CHO ratio was 0.66. For the business lot the average concentration for CH2O was 44 ppb, that for CH3CHO was 69 ppb, and the average CH2O/CH3CHO ratio was 0.65. The traffic in the parking facilities were light duty vehicles, using ethanol blended fuels for which CH3CHO emissions are more important. Sampling was done in a tunnel and the average CH2O concentration was 80 ppb, the average CH3CHO was 72 ppb, and the average CH2O/CH3CHO ratio was 1.2. This CH2O/CH3CHO ratio is consistent with a mix of 82% light duty and 18% heavy duty vehicles in the traffic counts for the tunnel. Sampling done in a residential area found an average CH2O/CH3CHO ratio of 1.0, while sampling done in a commercial area found an average ratio of 1.8. Sampling was also done in a rural area (Lagoa Verde) about 250 km from Salvador where the average CH2O/CH3CHO ratio was 0.8, and a fishing village (Cacha-Pregos) about 20 km from Salvador where the average ratio was 1.1. The authors concluded that the CH2O/CH3CHO ratio can be described fairly reliably by considering the vehicle fleet mix of heavy duty (diesel) vehicles and light duty (ethanol/gasohol) vehicles and the CH2O/CH3CHO ratio in emissions for these fuel types.44
Nguyen et al.47 have reported CH2O and CH3CHO measurements in São Paulo, Brazil and in Osaka, Japan. Aldehyde measurements were made in Osaka from May to December 1997, resulting in average CH2O and CH3CHO concentrations of 1.9 ppb and 1.5 ppb, and the average CH2O/CH3CHO ratio was 1.3. The São Paulo measurements were collected near the University of São Paulo campus on four days in February 1998. The average CH2O and CH3CHO concentrations were 5.0 ppb and 5.4 ppb respectively, and the average CH2O/CH3CHO ratio was 0.94. The use of the ethanol fuels in Brazil led to the higher CH3CHO concentrations relative to CH2O.
Grosjean et al.48 have reported CH2O and CH3CHO concentrations for an extensive set of measurements from Porto Alegre (April 1997–September 1999). From April 1997 to April 1999, 82% of the light duty vehicle fleet was fueled by gasoline mixed with 15% methyl tert-butyl ether (MTBE), while the remaining 18% was fueled by hydrous ethanol. In April 1999, the MTBE blended fuel was replaced by the conventional gasohol fuel used throughout the remainder of Brazil, a mixture of 20–25% ethanol and gasoline. Grosjean et al.48 had the unique opportunity to study the effects of this fuel change on the ambient atmospheric concentrations of CH2O and CH3CHO in Porto Alegre. As shown in Table 5, 3 h average concentrations were measured in the morning (0700–1000) and evening (1700–2000) periods both before and after the fuel change. The atmospheric concentrations are highly variable and do not show a large change, but the average CH2O/CH3CHO ratio does show a significant change from 2.26, when MTBE blended fuels were used, to 1.25 after the switch to gasohol for the morning samples, and a change from 1.75, when MTBE blended fuels were used, to 1.07 after gasohol fuels were used for the evening samples. These data make it clear that the CH3CHO emissions increase relative to CH2O emissions as the fuel changes from an MTBE blend to gasohol.
Montero et al.49 have reported measurements of CH2O and CH3CHO at two sites in São Paulo during August 1999. Two-hour samples were collected on consecutive days during morning (0800–1000), midday (1200–1400) and evening (1600–1800) time periods. The average CH2O/CH3CHO ratio varied with the time period of the day: for the Água Funda site the ratio was 0.9, 1.3, and 1.6 for the morning, midday and evening periods, while for the Cidade Universitária site the ratio was 0.7, 1.3 and 1.5 for these same periods. These data suggest that the morning measurements are predominately affected by direct vehicle emissions, while the midday and evening measurements have significant contribution from photochemically generated aldehydes as well. The CH2O/CH3CHO ratio is lowest in the morning sampling period, and increases as the day progresses. This is consistent with faster photochemical production of CH2O than CH3CHO.49
Correa et al.19,29 have collected samples for CH2O and CH3CHO at a site in Rio de Janeiro from December 1998 to December 2002. There was an attempt to look for trends in the annual average data. Insufficient samples were collected under similar conditions to allow identification of a trend. They suggested that CH2O concentrations increased and CH3CHO remained largely unchanged.29 This is consistent with the results of the small number of observations made in December through the years. Correa and Arbilla29 present data suggesting that the number of vehicles converted from gasohol to dual fueled with CNG increased from about 2% to about 12% of the fleet operating in Rio de Janeiro between 1998 and 2002 with the much higher CH2O/CH3CHO ratio in emissions for CNG vehicles as discussed earlier. This may be contributing to the increase in CH2O with relatively little change in CH3CHO.
Grosjean et al.50 collected a series of 3 h carbonyl samples during the morning rush hour from May to November 2000 at a downtown location in Rio de Janeiro. These samples were analyzed for up to 61 carbonyl compounds. The measurements were made at the FEEMA (state environmental agency in Rio de Janeiro) air monitoring site near Avenida Presidente Vargas. The average CH2O concentration for these measurements was 8.8 ppb, the average CH3CHO concentration was 5.8 ppb, and the average CH2O/CH3CHO ratio was 1.5. All of the other carbonyl compounds except acetophenone correlated well with CH2O and CH3CHO, suggesting similar sources. In this paper, it was noted that the CH2O/CH3CHO ratio has been increasing in more recent years. It was suggested that this is due to the fact that no new vehicles are being sold that run on pure ethanol, and that there is increasing use of gasohol fueled vehicles. This may change with higher ethanol content fuels used in flex fuel vehicles.
Vasconcellos et al.52 have reported the results of carbonyl measurements in tunnels and in the ambient air nearby during August and October 2001 in São Paulo. Only light duty vehicles are allowed to use the Janio Quadros Tunnel (JQT), while both light duty and heavy duty vehicles use the Maria Maluf Tunnel (MMT). Hence the JQT is used by gasohol and ethanol fueled vehicles and the MMT is used by gasohol, ethanol and diesel fueled vehicles. The CH2O/CH3CHO ratios in the tunnels averaged 0.9 for JQT and 1.0 for MMT and were slightly lower than the ambient samples near the tunnels which averaged 1.4. The higher CH2O/CH3CHO ratio in MMT than JQT is due to the larger fraction of emissions from the diesel vehicles allowed to operate in that tunnel.
Pinto and Solci53 have reported atmospheric measurements of CH2O and CH3CHO at two urban and one rural site in the area of Londrina (population about 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Londrina is located about 500 km west of São Paulo and about 800 km west of Rio de Janeiro. Measurements of CH2O and CH3CHO were reported for a central bus station site and at a downtown site during January and February 2002. The CH2O/CH3CHO ratio was 6.3 at the bus station, and 1.4 at the downtown site. The higher ratio at the bus station site is consistent with the much larger contribution of CH2O from diesel vehicles. Measurements were also reported for the downtown site and at a rural site during July 2002. The CH2O/CH3CHO ratio was 0.9 at the downtown site, and 2.5 at the rural site. This paper suggests that secondary sources are the most important for both aldehydes in rural areas, while primary sources are relatively important in urban areas.
000). Londrina is located about 500 km west of São Paulo and about 800 km west of Rio de Janeiro. Measurements of CH2O and CH3CHO were reported for a central bus station site and at a downtown site during January and February 2002. The CH2O/CH3CHO ratio was 6.3 at the bus station, and 1.4 at the downtown site. The higher ratio at the bus station site is consistent with the much larger contribution of CH2O from diesel vehicles. Measurements were also reported for the downtown site and at a rural site during July 2002. The CH2O/CH3CHO ratio was 0.9 at the downtown site, and 2.5 at the rural site. This paper suggests that secondary sources are the most important for both aldehydes in rural areas, while primary sources are relatively important in urban areas.
Martins et al.55 have reported atmospheric measurements of aldehydes in the Tijuca district of Rio de Janeiro between April 2002 and February 2003. A total of 86 samples were collected and analyzed for CH2O, CH3CHO, propionaldehyde, butyraldehyde and pentanaldehyde. The average concentrations of CH2O and CH3CHO were found to be 151 ± 64 ppb and 30 ± 14 ppb and the average CH2O/CH3CHO ratio was 4.9. The CH2O concentration and the CH2O/CH3CHO ratio are much higher than observed in most previous studies. The vehicular traffic in this area was mainly light duty vehicles which use hydrous ethanol (14.6%), gasohol (78%), and CNG (7.4%). Depending on the time of day, diesel fueled buses represent 7–20% of the fleet in this area. The higher CH2O concentrations relative to CH3CHO are believed to be due to the increased use of CNG as a fuel for light duty vehicles. The dominant aldehyde emitted from diesel fueled buses is also CH2O. These results are similar to those reported in another area of Rio de Janeiro during a similar time period by Rodrigues et al.54
de Andrade et al.61 reviewed data on atmospheric carbonyls through the 1990s. The range of concentrations measured for CH2O and CH3CHO in Brazil are similar to those measured elsewhere (largely in the US), but the average concentrations of both compounds are generally higher in Brazil. The differences between the measurements in Brazil and those elsewhere is more obvious in the CH2O/CH3CHO ratio which is often less than one, unlike that seen elsewhere in the world. This is due to the increased emissions of CH3CHO when ethanol is a fuel component.
Carvalho et al.62 have studied the biogenic emissions of HCs and carbonyl compounds from trees in forested areas of São Paulo. The emissions of CH3CHO from trees was the largest of all of the carbonyl compounds studied, being about 2.5 times the CH2O emission in one case, and no CH2O emission was detected in the other case. Biogenic contributions to carbonyl compounds in the atmosphere may be significant under some conditions.
The CETESB32 conducted an aldehyde measurement campaign between July and October 2006 at the Cerqueira César site in São Paulo. The average concentrations reported for the 141 samples of 2 h duration were 5.7 ppb for CH2O and 5.6 ppb for CH3CHO. These concentrations are somewhat lower than those reported at the same site during a similar time period in 1997, which found average concentrations of 7.0 ppb and 11.7 ppb, for CH2O and CH3CHO respectively.32 The analytical methodology changed, making the results for CH2O not directly comparable to the earlier results.
Jorge et al.63 have explored the relative importance of biogenic emissions of CH2O and CH3CHO from trees in forested areas of Rio de Janeiro. Samples were collected at four different sites: Praça Saens Peña, a heavy traffic area near the forest; the entrance to the forest; a scenic overlook in the forest and an additional interior site. The concentrations of CH2O and CH3CHO were highest where there was the most vehicle traffic. The CH2O/CH3CHO ratio decreased slightly at the lower traffic sites, possibly suggesting a slight increase in biogenic contributions to these compounds.
Some more recent papers29,54,55 have reported ambient data with CH2O/CH3CHO ratios that are high (4–5). Most of the earlier data have a CH2O/CH3CHO ratio of 1.5 or less. It is clear from these papers that the cause for the increase in the CH2O/CH3CHO ratio was an increase in ambient CH2O concentration, not a decrease in CH3CHO. The average CH2O is 68 ppb (n = 18),29 151 ppb (n = 86)54 and 132 ppb (n = 45)55 for 1.5–3 h average samples (Table 4). These very high concentrations have been observed at some sites in Rio de Janeiro, but not in the other areas. These concentrations are sufficiently high to be of significant concern. Some of the increase in CH2O concentration may be related to the increase in diesel fuel production relative to gasoline in Brazil, and to the increased use of CNG. Between 1998 and 2007, diesel fuel production in Brazil increased at a rate of about 2.0% year−1, while gasoline production only increased at a rate of about 0.4% year−1.64
Atmospheric ethanol
Schilling et al.65 developed a continuous enzymatic–fluorimetric method for the measurement of ethanol in ambient air. This method was used for ambient measurements of ethanol in Cubatão in the state of São Paulo during May 1996. Cubatão is located near the coast about 50 km southeast of the city of São Paulo. The diurnal character of the ethanol concentration was found to peak in the morning 0900–1000 at an average concentration of about 80 ppb and again in the evening at about 1800 at an average concentration of about 65 ppb. The diurnal average concentration of ethanol was found to remain relatively high (30–40 ppb) even through the nighttime hours. Table 6 shows a summary of these data and other measurements of methanol and ethanol in urban areas of Brazil.| Month/year | Location | Methanol/ppb | Ethanol/ppb | Number of samples | Sample period | Ref. |
|---|---|---|---|---|---|---|
| a nd—not detectable; na—not available. b Can—Summa canister grab sample; Sorbent—sorbent tube integrated sample. c 1, 2 and 4 h samples. d 1 and 2 h samples. e 1 h sample. | ||||||
| Rio de Janeiro | ||||||
| 1996–1997 | Muniz Barreto St. | 14.0 | 66.4 | 12 | 0.25–0.50 h | 66 |
| Jun. and Nov. 1999 | Eight urban tunnels | nda | 95.3 | 8 | 1 min | 69 |
| Jun. and Nov. 1999 | Four underground parking | nda | 371 | 4 | 1 min | 69 |
| Jun. 1999–May 2000 | Thirteen locations | nda | 26.4 | 13 | 1 min | 70 |
| May–Nov. 2000 | (FEEMA) Av. Presidente Vargas | nda | 16.4 | 6 | 1 min | 71 |
| Dec. 2003 | Eight locations | nda | 6.8 | 8 | 1 h | 58 |
| São Paulo | ||||||
| 1996–1997 | Rebouças | 19.6 | 36.2 | 9 | 0.25–0.50 h | 66 |
| Feb. 1998 | Univ. São Paulo | 34.1 | 176 | 11 | 1 min | 47 |
| Nov. 1998 | Cerqueira César | 9.2 | 468 | 6 | Canb,c | 68 |
| Nov. 1998 | Cerqueira César | nda | 11 | 16 | Sorbentb,c | 68 |
| Nov. 1998 | Ibirapuera Park | 12.9 | 397 | 6 | Canb,c | 68 |
| Nov. 1998 | Ibirapuera Park | 5.9 | 60 | 16 | Sorbentb,c | 68 |
| Nov. 1998 | Lapa | 13.4 | 313 | 6 | Canb,c | 68 |
| Nov. 1998 | Lapa | nda | 103 | 12 | Sorbentb,c | 68 |
| Nov. 1998 | Congonhas | 6.4 | 526 | 6 | Canb,c | 68 |
| Nov. 1998 | Congonhas | nda | 43 | 6 | Sorbentb,c | 68 |
| Nov. 1998 | Parque Dom Pedro | 6.9 | 138 | 4 | Canb,d | 68 |
| Nov. 1998 | Parque Dom Pedro | nda | 16 | 4 | Sorbentb,d | 68 |
| Nov. 1998 | Tunel 9 de Julho | 12.6 | 1018 | 2 | Canb,e | 68 |
| Nov. 1998 | Tunel 9 de Julho | nda | 105 | 2 | Sorbentb,e | 68 |
| Mar.–Apr. 2004 | Fourteen locations | nda | 13.9 | 14 | 1 h | 59 |
| Salvador | ||||||
| 1996–1997 | Garibaldi Av. | 9.8 | 65.4 | 21 | 0.25–0.50 h | 66 |
| 1996–1997 | Institute of Chemistry | nda (<7.6) | 17.9 | 5 | 0.25–0.50 h | 66 |
| Porto Alegre | ||||||
| Mar. 1996–Apr. 1997 | Rodoviaria | naa | 12.1 | 43 | 1 min | 67 |
| Apr. 1997 | Rodoviaria | naa | 31.6 | 6 | 1 min | 45 |
| Cubatão | ||||||
| May 1996 | Industrial complex | naa | 20–360 | Continuous (11 day) | 3 min | 65 |
| May 1996 | Industrial complex | naa | 30–80 | Diurnal average | 1 h | 65 |
Pereira et al.66 have developed a technique using sorbent cartridge collection of alcohols, followed by extraction and gas chromatography-flame ionisation detector (GC-FID) analysis. Ambient samples were collected on florisil cartridges at sites in Rio de Janeiro, São Paulo and Salvador. For all of these samples the ethanol concentrations are much higher than methanol.
Grosjean et al.45,67 have reported ambient measurements of ethanol for samples collected in Summa canisters in Porto Alegre between 1996 and 1997. The samples were analyzed by GC-mass spectrometry (MS) and GC-FID. During this period in the Porto Alegre area about 17% of the vehicles used hydrous ethanol fuel, 74% used a mixture of 85% gasoline and 15% MTBE and 9% were buses and trucks using diesel fuel without either ethanol or MTBE. All other areas of Brazil used gasohol instead of MTBE blended fuels. The ambient concentrations of ethanol ranged up to 68.2 ppb and averaged 12.1 ± 13.3 ppb.
Nguyen et al.47 have reported methanol, ethanol and isopropanol measurements by the alkyl nitrite formation reaction and product analysis by GC-electron capture detector (ECD) in São Paulo, Brazil and in Osaka, Japan. Alcohol measurements were made in Osaka every 2 h for one day a month from May to December 1997. The average methanol and ethanol concentrations were 5.8 ± 3.8 ppb and 8.2 ± 4.6 ppb respectively. Eleven samples were collected for alcohol analysis near the University of São Paulo campus on four days in February 1998. The average methanol and ethanol concentrations were 34.1 ± 9.4 ppb and 176.3 ± 38.1 ppb respectively. The average ratio of ethanol/methanol in São Paulo during the summer measurements was 5.2, while the average of the summer measurements of this ratio in Osaka was 0.9. The atmospheric concentrations of ethanol reported for São Paulo were about 20 times higher than those for Osaka. The use of ethanol fueled vehicles in São Paulo has led to extraordinarily high atmospheric concentrations of ethanol in the air.
Colon et al.68 collected and analyzed VOC samples from six different sites in São Paulo during November 1998. This was part of a joint USEPA and CETESB study of VOCs in São Paulo. For each sample day, samples were collected using sorbent tubes and also in Summa canisters. These samples were analyzed by GC-MS. It is believed that the alcohols were lost from the sorbent tubes during sample shipping prior to analysis. The methanol and ethanol concentrations were quantified in all of the canister samples. The VOC mix observed in São Paulo was dominated by ethanol. The methanol concentrations observed in the canisters in São Paulo are comparable to similar observations in the US, but the ethanol concentrations observed in São Paulo are about 40 times larger than previous observations in the US. This study should make the importance of measuring alcohol concentrations in the atmosphere quite clear.
Grosjean et al.69 have reported VOC and ethanol concentration data for canister grab samples collected in eight tunnels and four underground parking garages during June and November 1999 in Rio de Janeiro. These samples were analyzed by GC-MS. Grosjean et al.70 have reported VOC and ethanol concentration data for grab samples collected at 13 sites in Rio de Janeiro between June 1999 and May 2000. Grosjean et al.71 have reported VOC and ethanol concentration data for grab samples collected every two weeks at the FEEMA (state environmental agency) station near Avenida Presidente Vargas in Rio de Janeiro between May and November 2000.
Very limited data are available for ethanol concentrations in the atmosphere in Brazil. Ethanol concentrations of over 100 ppb were commonly reported in the urban areas where large quantities of ethanol were used.
Atmospheric BTEX
Gee and Sollars72 have reported some of the earliest measurements of benzene, toluene, ethylbenzene and xylenes (BTEX) compounds collected as 2 h samples on sorbent tubes and analyzed by GC-FID for São Paulo in 1995–1996. This study reported concentrations for several Latin American and Asian cities. Table 7 summarizes the measurements of the BTEX concentrations that have been reported for various urban areas in Brazil.| Month/year | Location | B/ppb | T/ppb | E/ppb | m,p-X/ppb | o-X/ppb | Number of samples | Sample period | Ref.a |
|---|---|---|---|---|---|---|---|---|---|
| a Rept—Report cited in numbered ref. b Total X—total xylenes. c nd—not detectable. d Can—Summa canister grab sample; Sorbent—sorbent tube integrated sample. e 1, 2 and 4 h samples. f 1 and 2 h samples. g 1 h sample. | |||||||||
| Rio de Janeiro | |||||||||
| Dec. 1995 | One location | 2.9 | 10.2 | 1.4 | Total Xb | 3.3 | 11 | — | 41 |
| Nov. 1996 | Praça Saens Peña | 1.7 | 3.9 | 0.7 | 2.4 | 0.9 | — | 1 h | Rept-55 |
| Dec. 1998–Mar. 1999 | Alto da Boa Vista | 0.1 | 0.6 | — | Total Xb | 0.0 | 3 | 24 h | 77 |
| Dec. 1998–Mar. 1999 | Ilha do Governador | 0.7 | 2.9 | — | Total Xb | 0.6 | 3 | 24 h | 77 |
| Dec. 1998–Mar. 1999 | Central business district | 1.0 | 3.6 | — | Total Xb | 0.7 | 3 | 24 h | 77 |
| Dec. 1998–Mar. 1999 | Brasil Ave. | 2.8 | 4.5 | — | Total Xb | 2.1 | 3 | 24 h | 77 |
| Jun. and Nov. 1999 | Eight urban tunnels | 11.8 | 17.9 | 5.0 | 12.4 | 4.4 | 8 | 1 min | 69 |
| Jun. and Nov. 1999 | Four underground parking | 27.8 | 64.7 | 11.1 | 26.8 | 9.5 | 4 | 1 min | 69 |
| Jun. 1999–May 2000 | Thirteen locations | 5.7 | 11.5 | 2.3 | 5.7 | 2.2 | 13 | 1 min | 70 |
| May–Nov. 2000 | (FEEMA) Av. Presidente Vargas | 3.5 | 7.6 | 2.0 | 3.8 | 1.4 | 13 | 1 min | 71 |
| Apr. 2002–Nov. 2002 | FIOCRUZ | 0.6 | 2.4 | 1.5 | 5.0 | 1.4 | 13 | 6 h | 54 |
| Apr. 2002–Feb. 2003 | Tijuca district | 0.3 | 1.3 | 0.8 | 2.4 | 0.7 | 30 | 6 h | 55 |
| Dec. 2003 | Eight locations | 1.8 | 8.7 | ndc | 4.2 | ndc | 8 | 1 h | 58 |
| Jun. 2004 | Service station | 45 | 42 | 8.2 | 28 | 11 | 6 | 2 min | 78 |
| Jun. 2004 | 200 m from Service stat. | 4.4 | 12 | 2.6 | 7.2 | 2.7 | 2 | 2 min | 78 |
| 2004–2005 | Av. Presidente Vargas | 4.5 | 6.1 | 2.1 | 4.4 | 2.0 | 94 | 6 h | 79 |
| Oct. 2005 | Rebouças Tunnel #1 | 9.1 | 12 | 2.8 | 8.3 | 1.8 | 5 | 1 h | 80 |
| Oct. 2005 | Rebouças Tunnel #2 | 25 | 32 | 7.6 | 22 | 5.3 | 5 | 1 h | 80 |
| São Paulo | |||||||||
| 1995–1996 | One location | 5.2 | 7.5 | 1.4 | 4.3 | 1.4 | — | 2 h | 72 |
| Nov. 1998 | Cerqueira César | 3.5 | 11.9 | 2.3 | 5.3 | 2.0 | 6 | Cand,e | 68 |
| Nov. 1998 | Cerqueira César | 0.8 | 6.4 | 2.0 | 5.8 | 2.0 | 16 | Sorbentd,e | 68 |
| Nov. 1998 | Ibirapuera Park | 1.4 | 7.4 | 1.4 | 2.5 | 0.9 | 6 | Cand,e | 68 |
| Nov. 1998 | Ibirapuera Park | 0.8 | 6.3 | 1.4 | 2.9 | 1.0 | 16 | Sorbentde | 68 |
| Nov. 1998 | Lapa | 1.4 | 11.7 | 3.1 | 6.0 | 1.6 | 6 | Cand,e | 68 |
| Nov. 1998 | Lapa | 1.5 | 12.2 | 3.6 | 7.1 | 1.8 | 12 | Sorbentd,e | 68 |
| Nov. 1998 | Congonhas | 3.5 | 6.5 | 1.3 | 4.6 | 1.6 | 6 | Cande | 68 |
| Nov. 1998 | Congonhas | 2.9 | 5.8 | 1.1 | 4.0 | 1.4 | 6 | Sorbentd,e | 68 |
| Nov. 1998 | Parque Dom Pedro | 0.9 | 4.4 | 0.8 | 1.6 | 0.5 | 4 | Cand,f | 68 |
| Nov. 1998 | Parque Dom Pedro | ndc | 1.5 | 0.5 | 1.4 | 0.4 | 4 | Sorbentd,f | 68 |
| Nov. 1998 | Tunel 9 de Julho | 11.0 | 13.7 | 3.0 | 10.8 | 4.1 | 2 | Cand,g | 68 |
| Nov. 1998 | Tunel 9 de Julho | 0.4 | 5.2 | 1.7 | 7.3 | 2.7 | 2 | Sorbentd,g | 68 |
| Aug. 2001 | Janio Quadros Tunnel | 13.9–24.8 | — | — | 21.6–36.4 | — | 3 | 5 min | 52 |
| Aug. 2001 | Ambient-Janio Quadros | 0.2–2.1 | — | — | 2.2–8.4 | — | — | 5 min | 52 |
| Oct. 2001 | Maria Maluf Tunnel | 13.7–16.6 | — | — | 21.4–24.8 | — | 6 | 5 min | 52 |
| Mar.–Apr. 2004 | Fourteen locations | 0.5 | 1.1 | 0.4 | 0.7 | — | 14 | 1 h | 59 |
| Porto Alegre | |||||||||
| Mar. 1996–Apr. 1997 | Rodoviaria | 3.7 | 5.5 | 1.3 | 3.2 | 1.3 | 46 | 1 min | 74 |
| Apr. 2002–Feb. 2003 | Background site | 0.1 | 0.2 | 0.05 | 0.1 | 0.05 | 2 | 1 min | 74 |
| Volta Redonda | |||||||||
| Dec. 1995–May 1996 | Five locations | 22 | 6.1 | — | Total Xb | 0.6 | 42 | 24 h | 73 |
| Apr.–May 1999 | Two locations | 7.2 | 1.1 | — | Total Xb | 0.1 | 11 | 24 h | 73 |
| Tubarão | |||||||||
| Dec. 1997 | Vila Moema | 1.8 | 0.6 | 0.1 | Total Xb | 0.5 | — | 24 h | 76 |
| Dec. 1997 | São Bernardo | 1.1 | 2.8 | 0.3 | Total Xb | 0.4 | — | 24 h | 76 |
| Capivari de Baixo | |||||||||
| Dec. 1997 | Capivari de Baixo | 1.2 | 1.8 | 0.1 | Total Xb | 0.2 | — | 24 h | 76 |
Gioda et al.73 have reported measurements of atmospheric concentrations of BTX compounds (ethylbenzene was not reported) in Volta Redonda city in the state of Rio de Janeiro. The largest steel producing facility in Brazil is located in Volta Redonda. The city is located about halfway between the cities of São Paulo and Rio de Janeiro. BTX concentrations were measured at five different sites during the first sampling (December 1995–May 1996) and at two different sites during the second sampling (April–May 1999). Certain industrial emissions control measures were implemented after the first sampling and prior to the second sampling.
Grosjean et al.74 have reported 48 ambient measurements of VOCs collected in electropolished stainless steel canisters between 20 March 1996 and 16 April 1997. Forty-six of these samples were collected in downtown Porto Alegre and two were collected at a background location. Grosjean et al.75 found strong correlations between each of the BTEX compounds and CO (correlation coefficients between 0.88 and 0.95). This suggests that these compounds arise largely from vehicle emissions in the Porto Alegre area.
dos Santos et al.76 have reported BTEX measurements at three sites in the cities of Tubarão and Capivari de Baixo in the state of Santa Catarina surrounding the largest coal-fired power plant in Latin America. The samples were 24 h average samples. The unusually high benzene/toluene ratio at the Vila Moema is believed to be due to the relatively larger emissions of benzene from the power plant.
Colon et al.68 collected and analyzed VOC samples from six different sites in São Paulo during November 1998. Samples were collected both in Summa canisters and with sorbent tubes. The BTEX concentrations observed in the canisters and sorbent tubes in São Paulo were similar. The mean concentrations of the BTEX compounds measured in São Paulo were 2–3 times higher than those measured in a similar USEPA study in the Los Angeles basin.
de Oliveira et al.78 reported measurements for 56 VOCs 200 m from a service station and 80 VOCs near the service station pumps during June 2004 in Rio de Janeiro. The concentrations of the BTEX compounds measured near the pumps were 3–4 times higher than those 200 m away, except for benzene which was about 10 times higher.
Correa and Arbilla79 have reported the results of an extensive set of atmospheric measurements of the BTEX compounds and two trimethylbenzenes at the Avenida Presidente Vargas site in the downtown area of Rio de Janeiro. Samples were collected on sorbent tubes for 94 days during 2004 and 2005 at this site. The samples were collected between 0900 and 1500, with 66 samples collected on Wednesdays, 13 on Thursdays and 15 on Fridays. The mean concentrations of O3, NOx and methane increased at the Avenida Presidente Vargas site between 2001 and 2004. The mean non-methane HC concentration remained largely unchanged during this period.
Machado et al.80 have reported concentrations of BTEX compounds in and near a light duty vehicle tunnel in Rio de Janeiro during October 2005. Five samples were collected at each of the two sites, one about 500 m from the tunnel inlet and the other about 1000 m further into the tunnel, about 500 m from its exit. The concentrations at the second sampling site were about 2.5 times those at the first. The mass ratio of benzene/toluene in this tunnel study was 0.62–0.66, which is similar to that found in tunnel studies in the US.
There are limited modern data for the BTEX compound concentrations in the urban areas of Brazil (Table 7). These data are not greatly different from those concentrations observed in many other areas of the world. One would expect the concentrations of the BTEX compounds from vehicle emissions to be lower than in other areas, due to the much greater fraction of ethanol in vehicle fuels in Brazil.
Atmospheric carboxylic acids
Andreae et al.81 have reported gaseous formic and acetic acid measurements made during the dry season (July–August 1985) in the central Amazon region of Brazil. Table 8 summarizes the measurements of formic and acetic acids made in Brazil. Talbot et al.82 reported 2.7 ± 1.4 ppb and 1.8 ± 1.0 ppb for formic and acetic acid (n = 50) for measurements made during the summer in the eastern US. The formic/acetic acid ratio was reported as 1.3 during the growing season and 0.9 during the non-growing season. From a tunnel study in the US, it was found that the mean ratio of formic/acetic acid in conventional vehicle exhaust was 0.5.82| Month/year | Location | Formic acid/ppb | Acetic acid/ppb | Formic/acetic acid ratio | Number of samples | Sample period/h | Ref. |
|---|---|---|---|---|---|---|---|
| Central Amazon | |||||||
| Jul.–Aug. 1985 | Ducke Forest Reserve | 1.4 | 1.8 | 0.6 | 14 | 0.25 | 81 |
| São Paulo | |||||||
| Jan. 1993 | Four sites | 3.8 | 3.6 | 1.1 | 4 | 3–6 | 83 |
| Sep. 1994 | Cidade Universitária | 4.6 | 1.4 | 3.4 | 7 | 8 | 84 |
| Jun. 1995 | Janio Quadros Tunnel | 5.5 | 17.2 | 0.3 | 1 | 3 | 84 |
| Jul. 1996 | Univ. São Paulo | 4.4 | 3.6 | 1.2 | 14 | 12 | 85 |
| Jul.–Sep. 1996 | Univ. São Paulo | 4.2 | 3.4 | 1.2 | 15 | 12 | 86 |
| Sep.–Nov. 1996 | Univ. São Paulo | 5.0 | 4.8 | 1.0 | 9 | 12 | 86 |
| Sep.–Oct. 1996 | CETESB downtown | 2.9 | 3.9 | 1.2 | 12 | 12 | 86 |
| Jul.–Aug. 1997 | CETESB downtown | 4.1 | 4.5 | 0.8 | 12 | 12 | 86 |
| Aug.–Sep. 1997 | Univ. São Paulo | 7.7 | 8.2 | 0.9 | 12 | 12 | 86 |
| Aug. 1999 | Água Funda | 6.2 | 3.1 | 2.0 | 32 | 2 | 49 |
| Aug. 1999 | Cidade Universitária | 10.2 | 2.8 | 3.6 | 35 | 2 | 49 |
| Aug. 2001 | Janio Quadros Tunnel | 2.4–4.3 | 12.8–20.9 | 0.2 | 6 | 2 | 52 |
| Aug. 2001 | Ambient-Janio Quadros | 3.1–18.4 | 0.5–6.4 | 2.7 | 12 | 2 | 52 |
| Oct. 2001 | Maria Maluf Tunnel | 5.2–7.5 | 8.5–16.0 | 0.5 | 6 | 2 | 52 |
| Oct. 2001 | Ambient-Maria Maluf | 0.6–19 | 1.9–10.6 | 4.3 | 12 | 2 | 52 |
| Araraquara | |||||||
| Apr. 1999–Mar. 2000 | Three sites | 9.0 | 1.3 | 6.9 | 33 | 3, 12, 24 | 87 |
| 1999 | Sugarcane burning | 2380 | 810 | 2.9 | 9 | 3 | 87 |
| Campinas | |||||||
| Jan. 1993 | Two sites | 1.9 | 3.6 | 0.6 | 2 | 6 | 83 |
Souza et al.85 have reported the results of ambient measurements of gas phase formic and acetic acid at the University of São Paulo during July 1996. Aerosol formic and acetic acids were also measured and it was found that about 85% of these acids are present in the gas phase. The formic/acetic acids ratio for these samples averaged 1.24 ± 0.26. It was found that the correlations between formic and acetic acids with CO were low, while the correlation coefficients between formic and acetic acids with O3 were 0.86 and 0.84. This suggests significant photochemical production of these organic acids. A related study at a more urban site found higher correlations between the acids and CO suggesting a greater significance of direct emissions.86
da Rocha et al.87 have reported formic and acetic acids at three sites in the state of São Paulo between April 1999 and March 2000. Three-hour , 12 h and 24 h samples were collected at an urban site in Araraquara on various days during the sampling period. Simultaneous samples were collected at the urban site, a semi-rural and a rural site near the city for ten days. The concentrations of formic and acetic acids measured at the three different sites were quite similar. Measurements were also made for these acids near burning and smouldering sugarcane. The concentrations were quite high, with formic acid ranging from 1160 to 4230 ppbv and acetic acid ranging from 360 to 1750 ppbv. During the biomass burning periods, the concentrations for formic and acetic acids measured at the urban site were 25% and 28% lower than during non-burning periods. Photochemical production of the acids is believed to be quite important.
Montero et al.49 have reported measurements of formic and acetic acids at two sites in São Paulo during August 1999. Two-hour samples were collected on consecutive days during morning (0800–1000), midday (1200–1400) and evening (1600–1800) time periods. The average concentrations of formic and acetic acids for the three different sampling periods of the day were about the same at both sites, as was the formic/acetic acid ratio. Good correlation between the formic acid and acetic acid concentrations was also observed at both sites.
Vasconcellos et al.52 have also reported the results of measurements of formic and acetic acids in tunnels in São Paulo. Measurements were made both in the tunnel and in the ambient air nearby. For the JQT (light duty vehicles only, August 2001) the formic/acetic acid ratio averages 0.2 in the tunnel and 2.7 in the ambient air near the tunnel. For the MMT (mixture of light duty and heavy duty vehicles, October 2001) the formic/acetic acid ratio averages 0.5 in the tunnel and 4.3 in the ambient air near the tunnel. The MMT tunnel ratio is higher due to the contribution of the acid emissions from the diesel vehicles, for which formic acid is the most abundant carboxylic acid.
The tunnel studies52,84 found a formic/acetic acid ratio of 0.2 and 0.3 for the JQT tunnel in São Paulo, which is restricted to light duty vehicles. Talbot et al.82 have reported a formic/acetic acid ratio of 0.5 for tunnel measurements in Hampton, Virginia. The tunnel data in Brazil suggest higher emissions of acetic acid than formic acid from the ethanol and gasohol fueled vehicles. Grosjean88 has concluded that reactions in the atmosphere produce more formic acid than acetic acid, which is consistent with the much larger formic/acetic acid ratio in ambient air. Many of the references have concluded that formic/acetic acid ratios less than one indicate a greater contribution from vehicle emissions, and ratios greater than one suggest greater importance of photochemical production of the acids.49,52,84–86
Ozone
Massambani and Andrade89 described the average and seasonal behavior of O3 in São Paulo during 1987. The O3 air quality standard in Brazil is 160 µg m−3 (81 ppb) as a 1 h average not to be exceeded more than once per year. During 1987, this standard was exceeded on 40 days. The diurnal average O3 concentration for the January–March quarter of the year showed a much higher peak O3 than observed in other quarters. The average peak O3 reached about 50 ppb in the afternoon (about 1400–1500). In the other quarters, the average peak O3 occurred about the same time of day, but only reached 25–30 ppb.Lazutin et al.90 reported results of O3 measurements made on the University of Campinas campus which is located about 10 km north of the center of the city of Campinas. During the measurement period which began in June 1993, the hourly averaged O3 concentration exceeded the O3 standard on five different days during August and early September. It is suggested that the high O3 episodes may be caused by photochemical transformations of pollutants from Campinas, from the city of São Paulo—which is about 100 km away, or from pollutants emitted from local biomass burning activities.
Onursal and Gautam12 discussed data for the 1995 measurements of O3 in São Paulo. The 1 h O3 standard was exceeded three times at Mooca, 30 times at Lapa and 20 times at Parque D. Pedro II, but was not exceeded at Congonhas. The highest concentration measured during 1995 was 389 ppb at Lapa. Ozone was monitored in the Tijuca area of Rio de Janeiro from October 1988 to January 1989, and the O3 standard was exceeded on four days.
Guardani et al.91 have studied atmospheric O3 measured during 1981–1996 at four monitoring sites in the São Paulo metropolitan area. The O3 standard was exceeded during each month of the year, with the largest number of O3 standard exceedances occurring between August and December. A neural network model for O3 was constructed using data from October–November 1996 and tested with data from the same months in 1997. In 1997, the O3 standard was exceeded on more than 100 days. The model was applied to hourly and daily predictions, and strong correlations between measured and modeled O3 concentrations were shown. Guardani and Nascimento92 have reported results of an extension of the neural network model for O3 to data collected from 1997 through 2001 at one of the air monitoring sites in the São Paulo metropolitan area. When the model is used to describe hourly average O3 concentrations, NO and NO2 concentrations are the most important variables, with radiation, temperature and relative humidity also being of importance. Guardani et al.93 have analyzed O3 data from the 12 O3 monitoring stations in the São Paulo area using data for 1997 through 2000. Principal component analyses were performed for three of the sites using data collected in 2000. At each site the first two components account for about 40% and 20% of the variability. At one site, the first component consisted of primary pollutants that are related to precursors for O3 formation, CO, NO, NO2, and NMHC. At the other two sites, the equivalent component was component 2 (NMHC was not measured at either of these sites, and CO was not measured at one of them). At the first site the second component included O3, temperature, radiation and relative humidity. This same set of variables was component 1 at the other two sites. The third and fourth components at each of the sites included wind speed and wind direction. The lack of measurements for all of the same quantities at each of the sites analyzed makes it more difficult to interpret the results of this work.
Muramoto et al.94 analyzed O3 data from eight sites in the São Paulo area from 1997 to 2001. They found that most high O3 events lasted only one or two hours, occurred mostly during the months of August–March, with peak O3 occurring between 1300 and 1600 and there was no day of week dependence to the high O3. The O3 air quality standard was exceeded on an average of about 10 days month−1 during these higher O3 months, and was exceeded during each month of the year.
Sanchez-Ccoyllo et al.95 used data from the June–August period of 1999 for the metropolitan area of São Paulo to try to identify important regions of O3 precursors by identifying the origins of back trajectories that lead to higher O3 concentrations. The same thing was done for PM10 and CO, both of which are primary pollutants and are much more stable than O3. The paper concluded that the highest O3 concentration was observed at a site towards the northeast edge of the metropolitan area, when the air arrived from the northeast. The authors concluded that long range transport was the important source for the O3 at this site. An alternative interpretation is that when the air originates in other quadrants it is much more likely to pass over the urban area where NO is being emitted, which would suppress O3 concentrations.
Tresmondi and Tomaz96 have studied the air pollution in the city of Paulínia in the state of São Paulo. Paulínia is the home of the largest petroleum refinery in Brazil and a number of chemical, pharmaceutical and fertilizer plants. But Paulínia is small, with a population of slightly over 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inhabitants and little downtown vehicle traffic. Paulínia is located about 110 km northwest of São Paulo and 20 km northwest of Campinas, both of which have a lot of vehicle traffic. The monitoring phase of the program extended from November 2000 to July 2002. The mobile monitoring station was located at four different sites in the Paulínia area during the program. Monitoring data were also available throughout the entire period at a downtown site operated by the CETESB. The mobile monitoring station was equipped to measure PM10, SO2–H2S, NO–NO2–NOx, O3, CO, CH4–NMHC, as well as meteorological sensors. During the 11 months of sampling at the Joao Aranha site (November 2000–September 2001) the 1 h average O3 standard was exceeded on two days, and on 16 days at the downtown Paulínia site during the same period. During the five months of sampling at the CPQBA site (October 2001–February 2002) the O3 standard was exceeded on 3 days, and on 5 days at the downtown Paulínia site. During the two months of sampling at the Parque Brasil 500 site (March–April 2002) and the three months of sampling at the Cosmopolis site (May–July 2002) the O3 standard was not exceeded, but at the downtown Paulínia site the O3 standard was exceeded on 3 days and 2 days respectively. High levels of O3 were not associated with a particular season, occurring both in summer and winter. Ozone is an important pollutant in Paulínia, and is believed to be formed mainly from precursors emitted in Campinas, because the prevailing winds blow from Campinas towards Paulínia.
000 inhabitants and little downtown vehicle traffic. Paulínia is located about 110 km northwest of São Paulo and 20 km northwest of Campinas, both of which have a lot of vehicle traffic. The monitoring phase of the program extended from November 2000 to July 2002. The mobile monitoring station was located at four different sites in the Paulínia area during the program. Monitoring data were also available throughout the entire period at a downtown site operated by the CETESB. The mobile monitoring station was equipped to measure PM10, SO2–H2S, NO–NO2–NOx, O3, CO, CH4–NMHC, as well as meteorological sensors. During the 11 months of sampling at the Joao Aranha site (November 2000–September 2001) the 1 h average O3 standard was exceeded on two days, and on 16 days at the downtown Paulínia site during the same period. During the five months of sampling at the CPQBA site (October 2001–February 2002) the O3 standard was exceeded on 3 days, and on 5 days at the downtown Paulínia site. During the two months of sampling at the Parque Brasil 500 site (March–April 2002) and the three months of sampling at the Cosmopolis site (May–July 2002) the O3 standard was not exceeded, but at the downtown Paulínia site the O3 standard was exceeded on 3 days and 2 days respectively. High levels of O3 were not associated with a particular season, occurring both in summer and winter. Ozone is an important pollutant in Paulínia, and is believed to be formed mainly from precursors emitted in Campinas, because the prevailing winds blow from Campinas towards Paulínia.
Boian and Kirchhoff97 reported O3 concentrations at a site near Maringa, a city in Paraná in the southern part of Brazil. These measurements were made from August 2001 to November 2002. During a five day period in late August 2001, the maximum O3 concentrations varied between 93 and 173 ppb, while the average concentration for the period was 89 ± 7.4 ppb. During this period, backward trajectory calculations suggested that the air mass originated in the northern part of Brazil. These northern areas of Brazil have relatively high levels of biomass burning during the dry season. More typical O3 concentrations during the dry season average about 47 ppb, while during January 2002 (the wet season) O3 averages about 13 ppb.
Martins et al.98 have analyzed air pollutant data for São Paulo between 1974 and 2002. There have been significant decreases in the concentrations of SO2, black smoke and CO over this time period. The concentration of NO2 has remained approximately constant between 1997 and 2002. The hourly average NO2 air quality standard (320 µg m−3) has been exceeded in each of these years, but only one or two times in the more recent years. The 24 h average PM10 standard (150 µg m−3) has been exceeded 20–60 days year−1 between 1998 and 2002. The CO concentrations have decreased over the years, but the 8 h average CO standard of 9 ppm continues to be exceeded mostly in the May–September period. The O3 air quality standard (1 h average of 160 µg m−3) has been exceeded during 116–219 days year−1 between 1997 and 2002. Even the WHO 8 h average O3 standard (120 µg m−3) has been exceeded during 51–151 days year−1 in this same period. The O3 air quality standard in the São Paulo metropolitan region was exceeded on 77 days in 2003, decreasing to 46 in 2006, and rebounding to 72 in 2007.14 A better understanding of the role of the O3 precursors in São Paulo is required to deal with the severe O3 pollution issues in the city. The O3 air quality standard is exceeded at essentially all of the 14 monitoring locations in the Rio de Janiero area.99 The O3 standard is exceeded more days than it is not at four of these monitoring locations.
Atmospheric PAN
PAN is formed downwind of major urban areas during periods of high photochemical activity. It is necessary that the ambient NO concentration be low compared to NO2. PAN is formed by the reaction of the peroxyacetyl radical with NO2. The reaction of CH3CHO with hydroxyl (OH) radicals and the photolysis of CH3CHO in air can lead to the production of the peroxyacetyl radical. Downwind of areas of high CH3CHO sources, one might expect to find high PAN concentrations.Tanner et al.37 have reported results of PAN and peroxypropionyl nitrate (PPN) measurements made using a GC equipped with an ECD. PAN and PPN were measured in 342 analyses at the Vila Isabel site in Rio de Janeiro from 1–8 July 1985, and in 135 analyses at the PUC/RJ site from 8–11 July 1985 (winter measurements). Strong diurnal trends in PAN concentrations were observed from 2–5 July and again on 10 July. The daytime maxima were over 2 ppb on each of these days. The highest concentration observed in these measurements was 5.4 ppb on 2 July. The PPN concentrations when detected were about 15–20% of the PAN concentrations. These PAN measurements were made in urban areas where fresh emissions of NO will suppress PAN formation.
Grosjean et al.100 have reported the results of a series of measurements of PAN and PPN in Santa Rita, about 20 km north–northwest of Porto Alegre, Brazil. The measurements were made by GC with an ECD during five periods: 25–31 May, 20–23 September, 30 September–6 October, 20 November–5 December 5, 1996 and 21–27 March, 1997. During the 1996–1997 period, about 74% of the vehicles used a mixture of 85% gasoline and 15% MTBE instead of gasohol as used elsewhere in Brazil. The average PAN concentration for the entire study period (952 observations) was 0.40 ± 0.63 ppb, the average daily maximum PAN was 1.44 ± 1.34 ppb (n = 41), and the maximum PAN measured throughout the study period was 6.67 ppb. These PAN concentrations are similar to those reported by Grosjean et al.100 in southern California between 1993 and 1997, and are lower than earlier PAN measurements in southern California. The CH3CHO concentration in Porto Alegre during this time period was among the lowest concentration reported in Brazil. In April 1999, the MTBE in Porto Alegre was replaced by ethanol as the oxygenated additive.
Grosjean et al.100 observed a fairly strong correlation between the daily maximum PAN and daily maximum O3 for the 41 days of measurements, R2 = 0.707. One goal of the research was to identify the important VOC precursors for PAN production in Porto Alegre, which requires extensive data for the ambient concentrations of VOCs in the area.74 An incremental reactivity approach was used to rank the importance of individual or lumped VOCs to PAN formation. The PAN formation potentials were calculated using the SAPRC 97 Version D chemical mechanism. The lumped VOCs—ARO2 (xylenes and trimethylbenzenes)—accounted for 16.7% of the PAN, 1,3-butadiene accounted for 12.5%, OLE2 (internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, such as cis-2-butene or trans-2-pentene) accounted for 11%, and isoprene accounted for 9.0%, while ethanol accounted for only 3.1% and CH3CHO for only 5.1% of the PAN. Ethanol and CH3CHO are expected to be less important precursors for PAN in Porto Alegre at this time, since less ethanol fuel was used than elsewhere in the country or in Porto Alegre after April 1999.
C bonds, such as cis-2-butene or trans-2-pentene) accounted for 11%, and isoprene accounted for 9.0%, while ethanol accounted for only 3.1% and CH3CHO for only 5.1% of the PAN. Ethanol and CH3CHO are expected to be less important precursors for PAN in Porto Alegre at this time, since less ethanol fuel was used than elsewhere in the country or in Porto Alegre after April 1999.
The PAN data in Brazil are quite limited. Much of the data has been collected in areas that have significant fresh vehicle emissions (high in NO), which suppresses PAN formation. This leaves considerable uncertainty as to the importance of PAN in Brazil.
Photochemical studies
Grosjean101 has reviewed the atmospheric chemistry of ethanol. The only important reaction for ethanol in the atmosphere is attack by the OH radical. The rate constant for this reaction is 3.3 × 10−12 cm3 molecule−1 s−1 at ambient temperatures. For a daytime average atmospheric OH radical concentration of 1 × 106 molecules cm−3, ethanol would have a half-life of about 2.4 days. The OH radical reaction proceeds by an H-atom abstraction from a C-atom. The H-atom abstraction from the O–H bond is negligible under atmospheric conditions. The more important routes for the OH reaction are:| OH + CH3CH2OH → H2O + CH3CHOH | (1a) |
| OH + CH3CH2OH → H2O + CH2CH2OH | (1b) |
| CH3CHOH + O2 → CH3CHO + HO2 | (2) |
| CH2CH2OH + O2 → OOCH2CH2OH | (3) |
| OOCH2CH2OH + NO → OCH2CH2OH + NO2 | (4) |
| OCH2CH2OH + O2 → HC(O)CH2OH + HO2 | (5) |
| OCH2CH2OH → CH2O + CH2OH | (6) |
| CH2OH + O2 → CH2O + HO2 | (7) |
| HO2 + NO → OH + NO2 | (8) |
Captive-air experiments
Akutsu et al.103 present some results of a few chamber experiments with pure compounds (methanol, ethanol, CH2O, or CH3CHO) and NOx mixtures, observing O3 production, HC loss and NOx concentration. A photochemical mechanism was developed and model calculations were performed to compare with these experiments. The experimental results were used for the validation of the mechanism. A series of model calculations were performed with this mechanism suggesting that at high CH2O/NOx ratios more O3 was produced than with propene, a high reactivity organic compound. Acetaldehyde photooxidation led to comparable quantities of O3 as did toluene, a moderately reactive organic. The photooxidation of methanol led to much less O3 production that butane, while the photooxidation of ethanol produced more O3, but still less than butane, a low reactivity organic molecule.Grosjean and Grosjean45 conducted six captive-air photochemical oxidation experiments in downtown Porto Alegre. In these experiments initial concentrations (ambient concentrations) of a number of pollutants were measured, including: CH2O average 15.8 ± 4.4 ppb, maximum 22.4 ppb; CH3CHO average 17.7 ± 7.2 ppb, maximum 31.3 ppb; MTBE average 9.1 ± 4.8 ppb, maximum 17.1 ppb; ethanol average 31.6 ± 22.4 ppb, maximum 68.2 ppb; benzene average 5.2 ± 2.3 ppb, maximum 8.0 ppb; toluene average 7.3 ± 3.2 ppb, maximum 12.2 ppb; ethylbenzene average 1.8 ± 0.9 ppb, maximum 3.3 ppb; m,p-xylene average 4.2 ± 1.9 ppb, maximum 7.1 ppb; and o-xylene average 1.7 ± 0.8 ppb, maximum 2.9 ppb. The captive photooxidation processes led to large increases in the CH2O and CH3CHO concentrations, by factors of 2.9 and 3.1 respectively, and large concentrations of both O3 (156–348 ppb) and PAN (13–30 ppb). These high concentrations were possible because no NO was being added to the system. The maximum PAN/maximum O3 ratio in these experiments was about 0.08–0.09. For an O3 episode in the Los Angeles area (8–9 September 1993), the maximum PAN/maximum O3 ratio in ambient air was 0.045–0.050 in central LA and 0.034–0.037 in Claremont.104,105 The higher PAN/O3 ratio in Brazil was likely due to the much higher CH3CHO concentrations and the large CH3CHO production. These captive-air experiments conducted with ambient air make the photochemical reactivity of the air, even in Porto Alegre, quite clear. Maximum incremental reactivity (MIR) factors106 were used to assess the relative importance of the oxidation of carbon containing precursors to O3 production. Ethene was most important followed by CO, CH3CHO, m,p-xylene and CH2O, while ethanol was ninth in the ranking and MTBE was twentieth. The presence of elevated concentrations of both CH3CHO and ethanol due to the ethanol fuel use appear to have significant effects on O3 production.
Pereira et al.107 conducted chamber experiments in November 2001 and March 2002 using zero-air mixed with high levels of fuel, either hydrous ethanol or gasohol and high levels of NO. Four sets of side by side experiments were run with each pair of fuels. Ambient irradiation occurred for periods of 5–7 h. In all cases the maximum O3 produced was higher with hydrous ethanol than with gasohol. The gasohol irradiations produced O3 quicker than ethanol, because the conversion of NO to NO2 was faster than with gasohol. Only O3 and NOx measurements were made during the course of these experiments. Clearly, ethanol can be photooxidized efficiently to produce O3.
Grosjean et al.108 have measured 150 VOCs in the ambient air of downtown Porto Alegre, including 46 alkanes, 30 alkenes, 22 aromatics, 17 carbonyls, 3 alcohols, and several other compounds. The authors used the ambient concentrations and the MIR values to calculate the O3 formation from the individual compounds present in the air. Ethene and CO had the largest O3 productions for the two ambient samples for which calculations were performed. Propene and m,p-xylene were the next two largest O3 producers, followed by 1,2,4-trimethylbenzene, toluene, trans-2-butene, and 2-methyl-2-butene. Formaldehyde and CH3CHO were either 10 and 9, or 10 and 12 in ranking for the two different samples. Ethanol ranked 35 and 19 in the two samples, and MTBE ranked 31 and 29. The vehicle fuels used in this area of Brazil during this time period were atypical of the fuels used elsewhere in the country, being a 15% blend of MTBE and gasoline instead of the normal Brazilian gasohol. Grosjean et al.109 evaluated the effects of switching from the 15% blend with MTBE to the conventional gasohol used throughout the remainder of Brazil. This change actually occurred in April 1999, and changes in the ambient VOC concentrations were used to estimate the change in the atmospheric reactivity towards O3 production. The analysis of the ambient data lead to the conclusion that the reactivity of the atmosphere decreased by about 4% when changing from the MTBE blended to the ethanol blended fuels. The reactivity decrease was due to the decrease in isobutene emissions from the MTBE blended fuel was almost exactly balanced by the reactivity increase due to the increased emissions of CH3CHO when the ethanol blended fuel is used.
Alvim et al.110 have measured 70 VOCs in the ambient air collected in 35 2-h average samples between 0600 and 1800 at the Cerqueira César air monitoring site in São Paulo during August and September 2006. The goal of this work was to identify the more important VOCs as precursors for O3 formation. Both the MIR approach and a photochemical box modeling approach were used to assess the importance of these compounds as O3 precursors. Based on the MIR approach, CH2O (5.58 ppb) and CH3CHO (5.04 ppb) ranked as the third and second most important VOCs, with 1-butene ranking first. Based on the photochemical box model CH2O and CH3CHO ranked as the first and fifth most important VOCs.
Photochemical modeling
Tanner et al.37 have reported results of photochemical modeling studies using a box model with time-varying dilution rates, primary emission rates, temperature and photolysis rates for the time period of the measurements (early July). The model was started at sunrise (∼0600) and ran for a 12 h period. The NO concentrations are relatively high. Both PAN and O3 concentrations will be strongly suppressed by this high NO. Acetaldehyde was found to vary between 30 and 60 ppb, PAN was found to peak at about 1400 at a concentration of about 4 ppb, and the maximum O3 was found to approach 50 ppb. The measurements that were made for CH3CHO and PAN are consistent with these model predictions.Pimentel and Arbilla111 have constructed a photochemical model for Rio de Janeiro considering the pollutants from alcohol fueled automobiles. The only carbon containing pollutants considered in this model were CO, CO2, CH2O and CH3CHO. The results suggested that the loss of CH2O by photolysis is 1.2–1.3 times faster than loss by reaction with OH radicals under the model conditions, while the loss of CH3CHO by reaction with OH is 15–18 times faster than loss by photolysis. Campos et al.112 present results of simple photochemical modeling of urban Rio de Janeiro using OZIPR with the SAPRC chemical mechanism.113 The model calculations were performed for the Avenida Brasil area of Rio de Janeiro for November 27, 1996. The model predicts a peak PAN concentration of about 0.6 ppb and peak O3 of about 23 ppb occurring at about 1500. The simulated O3 concentration is in good agreement with the measured O3. At this time of day the predicted NO concentration had dropped to about 50 ppb, which is sufficiently high to greatly suppress the concentrations of both PAN and O3. To form PAN, the peroxyacetyl radical must react with NO2 forming PAN, rather than reacting with NO that destroys the peroxyacetyl radical and prevents the formation of PAN. The effective rate constant ratio for the peroxyacetyl radical reacting with NO2 to that for it reacting with NO is about 0.4.114 This means that the concentration of NO2 needs to be about 2.5 times the concentration of NO for the PAN formation reaction to be equal in importance to the reaction with NO that prevents its formation. The model results predict that NO2 is only about 1.5 times the NO concentration when PAN peaks, and much less at all earlier times of day. Ozone concentrations are suppressed by the direct reaction of O3 with NO forming NO2. At an NO concentration of 50 ppb, the lifetime for O3 is only about 40 s. The NO2 that is formed by this reaction must be photolyzed to reform O3. In this model, the authors also present results for the same conditions, but with an MTBE blended gasoline substituted for the gasohol portions of the fuel used. The peak O3 for the MTBE blended fuel scenario increased to about 27 ppb, probably due to the small decrease in NOx emissions assumed for the fuel substitution (about 90% of the original NOx).
Arbilla and de Oliveira115 present the results of modifications to the SAPRC chemical mechanism113 to be appropriate for chemical modeling in Brazil. MTBE and ethanol chemistry was added to the mechanism. The model was tested by comparing the model results with measurements for NO, NO2, O3, and PAN from previous captive-air experiments.45 This model was also applied to real urban atmospheric conditions—high NO conditions. Arbilla et al.116 have reported results of model calculations for Avenida Presidente Vargas of Rio de Janeiro for December 1999. The maximum 1 h average O3 concentration at this high traffic site is calculated to be about 23 ppb, while the average maximum O3 for December was about 18 ppb. Model results were only compared with measurements for CO and O3. Correa et al.19 have reported additional results from this modeling. The simulated peak O3 was 23 ppb, while the measured maximum O3 for the month ranged between 5 and 35 ppb, averaging 19.1 ppb. The calculated CH2O and CH3CHO were found to peak shortly before noon, with CH2O about 14.8 ppb, CH3CHO about 21.7 ppb, and a CH2O/CH3CHO ratio of 0.67 near the peak concentrations.
Martins and Arbilla117 used this same model to evaluate the role of ethanol in the urban chemistry in Rio de Janeiro. Calculations were done for base-case conditions, which correspond to a relatively low ethanol concentration, initially about 12.4 ppb. During the early part of the day till about 1000, most of the CH2O and CH3CHO is primary, while later in the day most of these aldehydes are secondary. During the afternoon, about 50% of the CH2O is produced by the oxidation of alkenes and 1–7% is from the oxidation of ethanol. For CH3CHO, 85–92% is produced by the oxidation of alkenes during the afternoon and 4–7% by the oxidation of ethanol. A high ethanol case was also modeled which had the initial ethanol concentration at 250 ppb. The simulated concentrations of CH2O, CH3CHO and O3 all increased, and the CH2O/CH3CHO ratio decreased. These high and low ethanol cases are extreme. More typically, ethanol is between the extremes modeled in this work. The paper concludes that ethanol oxidation under the base-case conditions makes a relatively small contribution to CH3CHO formation.
Jaeschke et al.118 have reported elevated levels of CH3CHO in Brazil as a result of using ethanol fuel. They used a simplified photochemical model to evaluate some potential effects of using ethanol fuels in Germany. Model conditions appropriate for the Frankfurt-Griesheim station on 16 August 1994 were modeled using CH3CHO concentrations typical of the range of concentrations measured in Germany: 0.5 and 5.0 ppb. Additional calculations were performed which increased CH3CHO concentrations to 40 ppb, which might be typical with ethanol fuel use. Using the typical German conditions, the maximum PAN concentration was 1 ppb or less, but with the added CH3CHO the maximum PAN concentration increased to 8 ppb. Less significant effects were observed for O3. They concluded that the substitution of fossil fuel by ethanol “could lead to an increase of PAN concentrations with negative effects for plants and humans.”
de Freitas et al.119 discuss the construction and application of a simplified photochemical module which is incorporated into RAMS, a gridded model covering the São Paulo metropolitan area. The photochemical module consists of 15 reactions, with all VOCs (except CO) lumped in a single category. This model tended to overestimate the O3 concentrations.
Andrade et al.120 have shown that, using the base-case emissions inventory, the O3 modeled using the CIT model121 and SAPRC99 chemical mechanism significantly underestimated the O3 concentrations observed in São Paulo during a period in August 1999. Looking at ambient concentration data for NOx/CO the mass ratio was about 0.09, while the mass ratio of NOx/CO in the emissions inventory was 0.21. This suggests that NOx is overestimated or CO is underestimated in the inventory, or both. Model calculations were redone with NOx emissions reduced by a factor of 2, leading to significant increases in the predicted O3 concentration. The predicted NOx concentrations are still slightly higher than the measured concentrations and the predicted CO concentrations are significantly higher than the measured CO concentrations. There are undoubtedly still significant problems with the emissions inventory data used in the model.
Vivanco and Andrade122 present results of a top-down analysis (starting with ambient concentration data) to validate existing emission inventory data. Ambient data for early morning non-methane organic gas (NMOG) and NOxversus CO show good correlation. The top-down analysis suggests that the existing emissions inventory significantly overestimated NOx emissions. The revised emissions data were used with the CIT Eulerian photochemical model121 and applied to a three day period in August 1999. The calculated O3 concentrations were considerably higher and in better agreement with the measurements than were the results using the original emissions inventory. Similarly, the NO concentrations predicted using the revised emissions were lower and in better agreement with the measurements. The results for NO2 and NMOG concentrations did not change much as a result of application of the revised emissions inventory.
Sanchez-Ccoyllo et al.123 have presented model results for O3 using the CIT model121 and the SAPRC99 chemical mechanism. Calculations were performed for an episode on August 22, 2000. There is reasonable agreement between the measured and modeled results for O3, NO, CO, and reactive HCs (RHCs). To determine the effectiveness in RHC control versus NOx control, the O3 limitation was calculated:
| O3 limitation = O3 (−40% RHCanthro.) – O3 (−40% NOx) |
Sanchez-Ccoyllo et al.124 have presented additional model results for an O3 episode on 13–15 March 2000. Calculations were performed using base-case emissions for the metropolitan area. The agreement between the model results and measurements was good. Three additional vehicle emissions scenarios were also run. Scenario 1 assumed that all vehicles were operating within the PROCONVE guidelines (new vehicle emissions standards). Scenario 2 repeated the calculations assuming that the PROCONVE guidelines did not exist and vehicles were using pre-1989 technology. Scenario 3 assumed that no PROCONVE guidelines exist, but the progressive technological evolution in vehicle design occurred. The O3 predicted with Scenario 2 resulted in the highest O3. The O3 predicted for the base-case appears to be lowest, with slightly higher O3 for Scenarios 1 and 3. This appears to be the case, even though the O3 precursor emissions for Scenario 1 were lowest. There was no quantitative assessment of the O3 changes presented in this paper.
Martins and Andrade125 performed sensitivity studies of O3 formation potential. Ozone formation was found to be most sensitive to variations in the concentrations of four VOCs: butadiene, aromatics (kOH < 2 × 104 ppm−1 min−1), isoprene and CH2O. Martins and Andrade126 have extended this modeling research to evaluate the sensitivity of O3 concentration to individual VOCs during two different time periods in São Paulo. The top ten compounds or classes of compounds to which O3 was found to be the most sensitive included the reactive aromatic compounds, olefins (both classes), ethene, CH2O, toluene, ethanol, CO, reactive alkanes and CH3CHO.
Martins et al.127 have attempted to assess the impacts of biogenic VOC emissions (treated as isoprene and terpenes in the model) on the formation of O3, PAN and NOx in the urban sectors of São Paulo. It was found that including the biogenic emissions in the model could increase O3 by 6 or 15%, and increase PAN by 9 or 20% for two different modeling periods, as an average of model results for eight different monitoring sites. The effects on the NO and NO2 concentrations were much smaller.
Rodrigues et al.54 have simulated pollutant concentrations for an August 2003 day for the Manguinhos campus of FIOCRUZ in Rio de Janeiro. The SAPRC chemical mechanism113 was used on this work. Measured average concentrations for CO and O3 at this site are compared with the simulated results. This suburban site is characterized by a high VOC/NOx ratio (16.7), which is significantly different from earlier simulations at the Avenida Presidente Vargas site that has high vehicular traffic density and a low VOC/NOx ratio (2.5). No results are presented for the simulated concentrations of CH2O, ALD2 (CH3CHO), TOLU (toluene and ethylbenzene) or XYLE (total xylenes) which could be compared with the measured values. Such a comparison would have been most useful for CH2O and CH3CHO, to assess how well emissions and photochemical production of these compounds are being dealt with in these simulations.
Dallarosa et al.128 have simulated the air in the vicinity of the Candiota coal-fired power station for the periods of January 24–26, 2003 and August 10–12, 2004. The model predicts very high O3 concentrations (>200 ppb) during the January modeling period. The predicted concentrations during the August period are much lower (<60 ppb). Measured and modeled concentrations of NO and NO2 were compared at three monitoring sites. There were significant differences between the measured and modeled results. These model results are not affected significantly by vehicle emissions, due to the selection of the model domain.
Conclusions
Faiz et al.129 have assessed the importance of motor vehicle emissions on air pollution issues in Latin American countries. In São Paulo in 1990, the motor vehicle contributions to CO, HC and NOx account for 89–94% of the ambient concentrations. The use of alcohol based fuels in Brazil has led to increased emissions of aldehydes and unburned ethanol, which are commonly included in estimates of HC emissions. About 25% of Brazil's automobile fleet operates in the São Paulo metropolitan area.Bell et al.130 have concluded that there are severe air quality problems in Latin America. In their assessment of O3 and particulate matter pollution problems in São Paulo, they concluded that over 113![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 adult and 735 infant deaths could be avoided between 2000 and 2020 by implementation of a control policy scenario that would lead to a gradual decrease in the annual average PM10 concentration by 5.2 µg m−3 and in the annual average daily 1 h maximum O3 concentration by 4.2 ppb by 2020 in São Paulo. There would also be large decreases in morbidity, restricted activity days and work loss days resulting from this control policy as compared to business as usual.
000 adult and 735 infant deaths could be avoided between 2000 and 2020 by implementation of a control policy scenario that would lead to a gradual decrease in the annual average PM10 concentration by 5.2 µg m−3 and in the annual average daily 1 h maximum O3 concentration by 4.2 ppb by 2020 in São Paulo. There would also be large decreases in morbidity, restricted activity days and work loss days resulting from this control policy as compared to business as usual.
Andrade et al.131 have evaluated the potential air quality impacts of several emissions control strategies on the air quality in São Paulo. The most aggressive emissions control scenario involved a São Paulo integrated transport plan increasing public transport from 50.7% in 2000 to 57% in 2010 with increased use of subways and trains, greater use of alternative buses (natural gas and hybrid diesel–electric), implementation of an effective inspection and maintenance program for vehicles, greater use of “best” diesel trucks for road cargo (totally replacing the fleet by 2020), and changes in the commercial and industrial sector with greatly increased use of natural gas instead of other fossil fuels across the sector. Under this scenario, CO is expected to decrease by 18% in 2020 relative to 2000, with O3 increasing by 32%, NO increasing by 36%, and NO2 increasing by 40% by 2020. During this same period, SO2 is predicted to decrease by 47% and PM10 would decrease by 45%. The diesel truck replacement is largely responsible for the benefits for PM10. All of the segments of the emissions control scenario have some benefits for O3, relative to business as usual approaches, but none of the tested scenarios lead to a smaller increase in O3 than the 32% of the aggressive combination of actions. The continued growth in NOx and O3 is due to the continued growth in industrial and transportation sources and the extent and rate at which the proposed changes can be implemented.
It is quite clear that there is a need to decrease the use and dependence on fossil fuels. Much of the basic transportation system of the world is based on vehicles that are fueled by liquid fuels. This was the basis for the development of ethanol for fuel use in Brazil many years ago. Clearly, ethanol and ethanol–gasoline blends can be used as the much needed liquid fuel that can relatively easily be adapted for vehicle use. These fuels have the added benefit of being renewable fuels or biofuels, which may aid in dealing with global climate impacts. Vehicle technology has been developed and continues to evolve to make optimal use of gasoline and diesel fuels. Light duty gasoline fueled vehicles are optimized to maximize performance characteristics of the vehicle while minimizing the emissions of CO, VOCs, NOx, and PM. For the most part, we ignore the hundreds of individual compounds that are actually emitted from the vehicle in that broad range of VOC compounds. As fuel composition changes, it is necessary to look at the details of the VOCs and how they change with changing fuel composition. If we do not, there can be dramatic effects on air quality, as we have seen in Brazil.
Gaffney and Marley132 have pointed out that the “combustion of renewable fuels may, in some cases, result in a reduction in the criteria pollutants, the emissions may contain significant amounts of currently unregulated yet equally important pollutants.” The use of ethanol blended fuels leads to an increase in the emissions of ethanol and CH3CHO which will increase the photochemical reactivity and toxicity of the emissions. Vehicle technology must be driven to deal effectively with these adverse changes in emissions, before there is widespread use of different fuel types. The use of high ethanol content fuels will lead to a large increase in the emissions of both ethanol and CH3CHO. If ethanol is oxidized too slowly to have significant impacts on urban O3, it will still be oxidized, largely through CH3CHO. The use of these fuels may have significant impacts on the global atmospheric sources of both ethanol and CH3CHO.4 This could lead to significant impacts on the regional and global oxidants: O3 and PAN.
Flex fuel vehicles have been developed that operate on varying composition mixtures of ethanol and gasoline. But flex fuel vehicles in Brazil mean something very different from those being developed in much of the rest of the world. In Brazil, flex fuel vehicles are designed to operate using fuels that range from gasohol (in Brazil 20–25% anhydrous ethanol in gasoline) to hydrous ethanol (approximately 95.5% ethanol with the rest being water), and any mixture of these fuels. In North America, the flex fuel vehicles are designed to operate using fuels that range from gasoline (no ethanol) to E85 (85% anhydrous ethanol and 15% gasoline), and any mixture of these fuels. These are very different fuels that are required to operate reliably under very different conditions. Clearly the initial development of these vehicles has focused on reliable and economical operation of these vehicles. More attention needs to be paid to emissions control issues. There arevery little data in the literature dealing with identifying, quantifying and reducing the emissions of the unregulated pollutants from these vehicles.
Bravo et al.133 have discussed the impact of the phase-out of lead in gasoline in Mexico on the air quality in Mexico City. This is very clearly an important change that must be made in the world. The use of reformulated gasoline (RFG) has been touted as a means of reducing urban air pollution problems. The elimination of lead in vehicle fuels and use of RFGs was intended to reduce lead while boosting the octane rating for the fuel. The old vehicle fleet in Mexico lacks the emissions controls that will reduce RHC emissions. The program led to a significant decrease in atmospheric lead concentrations and a significant increase in O3 problems in Mexico City. This is an example of what can happen when one makes a necessary change in a vehicle fuel, that has worked successfully in one area of the world, and applies it in another, without thinking about and analyzing the other impacts of this change. These are the same kinds of issues that will arise if one tries to quickly initiate the use of ethanol fuels with high ethanol content in other areas of the world, particularly in the developing countries of Latin American, Africa and Asia.
Current motor vehicles are not likely to function with optimal efficiency with a significant change in vehicle fuel composition. These vehicles will need to be modified to function optimally, both for vehicle efficiency and emissions. To deal with vehicle emissions, this will require extensive testing of the new fuels in vehicles with and without modifications to find the most appropriate emissions controls. The emissions that are monitored in this testing must be broadened to include the many specific pollutants that are of increased significance from the new fuels. In the case of high ethanol content fuels, in addition to information about changes in individual VOCs that are related to gasoline fuel use, increased emphasis must be placed on the emissions of carbonyl compounds, both CH3CHO and CH2O, and on ethanol emissions. The control technologies will need to be modified to reduce the emissions of these significantly altered pollutants. At the same time these fuel development and vehicle emissions control issues are being studied, the air quality monitoring programs in urban areas that may be impacted by the changed vehicle emissions must be modified. There must be increased monitoring for the specific pollutants that are expected to change significantly with the use of the new fuels. This will provide baseline data to allow one to better understand the measurements of ambient concentrations of CH3CHO and ethanol that might be made in the air after the new fuels are in widespread use. This systematic approach to developing more appropriate emissions controls for the new fuels and to air quality monitoring will make it easier to identify problems and to solve them when they inevitably occur.
Jacobson134 has recently reviewed energy related solutions to global warming, air pollution and energy security. In that work, it was concluded that battery-electric vehicles are much more likely to be a better long-term solution to our transportation needs than are liquid fuel vehicles. Developing this technology sufficiently for widespread application is still well in the future. Liquid fuel vehicles will continue to be used for many years.
References
- M. Dickerson, The Seattle Times, June 17, 2005, http://seattletimes.nwsource.com/html/nationworld/2002339093_brazilfuel17.html Search PubMed.
- R. K. Niven, Renewable Sustainable Energy Rev., 2005, 9, 535–555 CrossRef CAS.
- J. Fargione, J. Hill, D. Tillman, S. Polasky and P. Hawthorne, Science, 2005, 319, 1235–1238.
- M. Z. Jacobson, Environ. Sci. Technol., 2007, 41, 4150–4157 CrossRef CAS.
- O. M. Brilhante, J. Environ. Plann. Management, 1997, 40, 435–449 Search PubMed.
- J. Martines-Filho, H. L. Burnquist and C. E. F. Vian, Choices, 2006, 21, 91–96 Search PubMed.
- J. Goldemberg, Environ. Res. Lett., 2006, 1, 014008 Search PubMed.
- F. Rosillo-Calle and L. A. B. Cortez, Biomass Bioenergy, 1998, 14, 115–124 CrossRef CAS.
- J. R. Moreira and J. Goldemberg, Energy Policy, 1999, 27, 229–245 CrossRef.
- L. Szwarcfiter, F. E. Mendes and E. L. La Rovere, Trans. Res. Part D, 2005, 10, 153–160 Search PubMed.
- A. Szwarc, III Encontro Internacional AFEEVAS, Brasilia, 2004, http://www.afeevas.org.br/afeevas/PDF/Semin%E1rio%20AFEEVAS%20Jun2004%20%5BSomente%20leitura%5D.pdf Search PubMed.
- B. Onursal and S. P. Gautam, Vehicular air pollution: Experiences from seven Latin American urban centers,The World Bank, Washington, DC, 1997 Search PubMed.
- I. Romieu and M. Lacasana, in Lead in the Americas: A Call for Action, ed. C. P. Howson, M. Hernandez-Avila and D. P. Rall, U. S. National Academy of Sciences, Washington, DC, U. S. A., 1996, pp. 77–83 Search PubMed.
- CETESB, Relatório de Qualidade do Ar no Estado de São Paulo 2007, São Paulo, Brazil, 2008, http://www.cetesb.sp.gov.br/Ar/relatorios/RelatorioAr2007.zip Search PubMed.
- J. B. de Andrade and A. H. Miguel, Int. J. Environ. Anal. Chem., 1985, 21, 229–237.
- J. B. de Andrade and A. H. Miguel, Quim. Nova, 1985, 8, 356–357 CAS.
- A. H. Miguel and J. B. de Andrade, J. Braz. Chem. Soc., 1990, 1, 124–127 CAS.
- M. X. Coutrim, L. A. Nakamura and C. H. Collins, Chromatographia, 1993, 37, 185–190 CAS.
- S. M. Correa, E. M. Martins and G. Arbilla, Atmos. Environ., 2003, 37, 23–29 CrossRef CAS.
- L. C. M. Sales and J. R. Sodre, Combust. Sci. Technol., 2003, 175, 1535–1550 CrossRef CAS.
- O. Lucon, O. M. Alvares and S. T. Coelho, International Symposium on Alcohol Fuels XV, San Diego, CA, USA, 2005, http://www.eri.ucr.edu/ISAFXVCD/ISAFXVPP/BWF.pdf Search PubMed.
- R. de Abrantes, J. V. de Assuncao, C. R Pesquero, R. E. Bruns and R. P. Nobrega, Atmos. Environ., 2009, 43, 648–654 CrossRef CAS.
- R. de Abrantes, J. V. de Assuncao and E. Y. Hirai, Rev. Saude Publica, 2005, 39, 479–485 Search PubMed.
- R. de Abrantes, J. V. de Assuncao and E. Y. Hirai, Rev. Saude Publica, 2005, 39, 1–6 Search PubMed.
- S. M. Correa and G. Arbilla, Atmos. Environ., 2006, 40, 6821–6826 CrossRef CAS.
- S. M. Correa and G. Arbilla, Atmos. Environ., 2008, 42, 769–775 CrossRef.
- L. L. N. Guarieiro, P. A. F. Pereira, E. A. Torres, G. O. da Rocha and J. B. de Andrade, Atmos. Environ., 2008, 42, 8211–8218 CrossRef CAS.
- P. M. Merritt, V. Ulmet, R. L. McCormick, W. E. Mitchell and K. J. Baumgard, SAE Brasil Fuels and Lubricants Meeting, Paper No. 2005-01-2193, Rio de Janiero, Brazil, 2005, http://www.nrel.gov/vehiclesandfuels/npbf/pdfs/38493.pdf Search PubMed.
- S. M. Correa and G. Arbilla, Atmos. Environ., 2005, 39, 4513–4518 CrossRef CAS.
- L. D. Martins, M. F. Andrade, E. D. Freitas, A. Pretto, L. V. Gatti, E. L. Albuquerque, E. Tomaz, M. L. Guardani, M. H. R. B. Martins and O. M. A. Junior, Environ. Sci. Technol., 2006, 40, 6722–6729 CrossRef CAS.
- E. L. Teixeira, S. Feltes and E. R. R. de Santana, Quim. Nova, 2008, 31, 244–248 CAS.
- CETESB, Relatório de Qualidade do Ar no Estado de São Paulo 2006, São Paulo, Brazil, 2007, http://www.cetesb.sp.gov.br/Ar/relatorios/RelatorioAr2006.zip Search PubMed.
- G. L. Domingues and L. V. Gatti, 48th Congresso Brasileiro de Quimica, Rio de Janiero, Brazil, 2006, http://www.abq.org.br/cbq/2008/trabalhos/5/5-502-4748.htm Search PubMed.
- I. Romieu, H. Weitzenfeld and J. Finkelman, J. Air Waste Manage. Assoc., 1991, 41, 1166–1171 CAS.
- B. R. Gurjar, T. M. Butler, M. G. Lawrence and J. Lehieveld, Atmos. Environ., 2008, 42, 1593–1606 CrossRef CAS.
- R. Godinho, W. Acquaviva and J. Romano, 11th Brazilian Congress for Environmental and Sanitation Engineering, Fortaleza, 1981 Search PubMed.
- R. L. Tanner, A. H. Miguel, J. B. de Andrade, J. S. Gaffney and G. E. Streit, Environ. Sci. Technol., 1988, 22, 1026–1034 CAS.
- D. C. Lowe and U. Schmidt, J. Geophys. Res. C: Oceans Atmos., 1983, 88, 10844–10858 CAS.
- D. Grosjean, A. H. Miguel and T. M. Tavares, Atmos. Environ., 1990, 24B, 101–106 CAS.
- A. H. Miguel, F. R. A. Neto, J. N. Cardoso, P. C. Vasconcellos, A. S. Pereira and K. S. G. Marquez, Environ. Sci. Technol., 1995, 29, 338–345 CAS.
- J. B. de Andrade, H. L. C. Pinheiro and M. V. Andrade, J. Braz. Chem. Soc., 1995, 6, 287–290 CAS.
- L. S. R. Brickus, J. N. Cardoso and F. R. A. Neto, Environ. Sci. Technol., 1998, 32, 3485–3490 CrossRef CAS.
- A. Gioda and F. R. A. Neto, Quim. Nova, 2003, 26, 359–365 CAS.
- J. B. de Andrade, M. V. Andrade and H. L. C. Pinheiro, J. Braz. Chem. Soc., 1998, 9, 219–223 CAS.
- E. Grosjean and D. Grosjean, J. Braz. Chem. Soc., 1998, 9, 131–143 CAS.
- M. Pires and L. R. F. de Carvalho, Quim. Nova, 1999, 22, 487–496 CAS.
- H. T.-H. Nguyen, N. Takenaka, H. Bandow, Y. Maeda, S. T. de Oliva, M. M Botelho and T. M. Tavares, Atmos. Environ., 2001, 35, 3075–3083 CrossRef CAS.
- E. Grosjean, D. Grosjean, M. T. R. Rodriguez and L. F. R. Moreira, 94th Air and Waste Management Association Annual Meeting, Paper No. 378, Orlando, Florida, 2001 Search PubMed.
- L. Montero, P. C. Vasconcellos, S. R. Souza, M. A. F. Pires, O. R. Sanchez-Ccoyllo, M. F. Andrade and L. R. F. Carvalho, Environ. Sci. Technol., 2001, 35, 3071–3081 CrossRef CAS.
- D. Grosjean, E. Grosjean and L. F. R. Moreira, Environ. Sci. Technol., 2002, 36, 1389–1395 CrossRef CAS.
- F. R. Rocha, L. H. G. Coelho, M. L. A. Lopes, L. R. F. Carvalho, J. A. F. da Silva, C. L. do Lago and I. G. R. Gutz, Talanta, 2008, 76, 271–275 CrossRef CAS.
- P. C. Vasconcellos, L. R. F. Carvalho and C. S. Pool, J. Braz. Chem. Soc., 2005, 16, 1210–1216 CAS.
- J. P. Pinto and M. C. Solci, J. Braz. Chem. Soc., 2007, 18, 928–936 CAS.
- F. Rodrigues, I. Milas, E. M. Martins, G. Arbilla, G. F. Bauerfeldy and M. de Paula, J. Braz. Chem. Soc., 2007, 18, 342–351 CAS.
- E. M. Martins, G. Arbilla, G. F. Bauerfeldt and M. de Paula, Chemosphere, 2007, 67, 2096–2103 CrossRef CAS.
- R. M. Cavalcante, B. H. Seyffert, M. G. M. D'Oro, R. F. Nascimento, C. S. Campelo, I. S. Pinto, F. B. Anjos and A. H. R. Costa, Indoor Built Environ., 2005, 14, 165–172 Search PubMed.
- R. M. Cavalcante, C. S. Campelo, M. J. Barbosa, E. R. Silveira, T. V. Carvalho and R. F. Nascimento, Atmos. Environ., 2006, 40, 5701–5711 CrossRef CAS.
- A. Moreira, J. C. Almeida, A. S. Minho, D. Grosjean, E. Grosjean and R. A. Rasmussen, 98th Air & Waste Management Association Annual Meeting, Paper No. 1091, Minneapolis, Minnesota, 2005 Search PubMed.
- A. Moreira, J. C. Almeida, A. S. Minho, D. Grosjean, E. Grosjean and R. A. Rasmussen, 98th Air & Waste Management Association Annual Meeting, Paper No. 1102, Minneapolis, Minnesota, 2005 Search PubMed.
- E. D. Sodre, S. M. Correa, G. Arbilla and M. Marques, Quim. Nova, 2008, 31, 249–253 CAS.
- M. V. A. S. de Andrade, H. L. C. Pinheiro, P. A. P. Pereira and J. B. de Andrade, Quim. Nova, 2002, 25, 1117–1131.
- L. R. F. Carvalho, P. C. Vasconcellos, W. Mantovani, C. S. Pool and S. O. Pisani, J. Environ. Monit., 2005, 7, 493–499 RSC.
- D. C. Jorge, L. V. da Silva and G. Arbilla, 48th Congresso Brasileiro de Quimica, Rio de Janiero, Brazil, 2006, http://www.abq.org.br/cbq/2008/trabalhos/5/5-238-4556.htm Search PubMed.
- A N P–Agencia Nacional do Petroleo, Gas Natural e Biocombustiveis, Table 3.2, http://www.anp.gov.br/conheca/anuario_2008.asp.
- M. Schilling, G. Voigt, T. Tavares and D. Klockow, Fresenius’ J. Anal. Chem., 1999, 364, 100–105 CrossRef CAS.
- P. A. P. Pereira, E. T. S. Santos, T. F. Ferreira and J. B. de Andrade, Talanta, 1999, 49, 245–252 CrossRef.
- E. Grosjean, D. Grosjean, R. Gunawardena and R. A. Rasmussen, Environ. Sci. Technol., 1998, 32, 736–742 CrossRef CAS.
- M. Colon, J. D. Pleil, T. A. Hartlage, M. L. Guardani and M. H. Martins, Atmos. Environ., 2001, 35, 4017–4031 CrossRef CAS.
- D. Grosjean, E. Grosjean, R. A. Rasmussen, L. F. R. Moreira and A. Moreira, 96th Air & Waste Management Association Annual Meeting, Paper No. 69181, San Diego, California, 2003 Search PubMed.
- D. Grosjean, E. Grosjean, R. A. Rasmussen, L. F. R. Moreira and A. Moreira, 96th Air & Waste Management Association Annual Meeting, Paper No. 69182, San Diego, California, 2003 Search PubMed.
- D. Grosjean, E. Grosjean, R. A. Rasmussen, L. F. R. Moreira and A. Moreira, 96th Air & Waste Management Association Annual Meeting, Paper No. 69185, San Diego, California, 2003 Search PubMed.
- I. L. Gee and C. J. Sollars, Chemosphere, 1998, 36, 2497–2506 CrossRef CAS.
- A. Gioda, J. A. Sales, P. M. S. Cavalcanti, M. F. Maia, L. F. P. G. Maia and F. R. A. Neto, J. Braz. Chem. Soc., 2004, 15, 856–864 CAS.
- E. Grosjean, D. Grosjean and R. A. Rasmussen, Environ. Sci. Technol., 1998, 32, 2061–2069 CrossRef CAS.
- E. Grosjean, R. A. Rasmussen and D. Grosjean, Environ. Sci. Technol., 1999, 33, 1970–1978 CrossRef CAS.
- C. Y. M. dos Santos, D. A. Azevedo and F. R. A. Neto, Atmos. Environ., 2004, 38, 1247–1257 CrossRef.
- M. B. Fernandes, L. S. R. Brickus, J. C. Moreira and J. N. Cardoso, Chemosphere, 2002, 47, 417–425 CrossRef CAS.
- K. M. P. G. de Oliveira, E. M. Martins, G. Arbilla and L. V. Gatti, Bull. Environ. Contam. Toxicol., 2007, 79, 237–241 CrossRef CAS.
- S. M. Correa and G. Arbilla, J. Braz. Chem. Soc., 2007, 18, 539–543 CAS.
- M. C. S. Machado, J. C. S. Aimeida, E. M. Martins and G. Arbilla, Bull. Environ. Contam. Toxicol., 2007, 78, 304–307 CrossRef CAS.
- M. O. Andreae, R. W. Talbot, T. W. Andreae and R. C. Harriss, J. Geophys. Res., 1988, 93D, 1616–1624 CrossRef.
- R. W. Talbot, K. M. Beecher, R. C. Harriss and W. R. Cofer, J. Geophys. Res., 1988, 93D, 1638–1652 CrossRef.
- A. G. Allen and A. H. Miguel, Atmos. Environ., 1995, 29, 3519–3526 CrossRef CAS.
- S. R. Souza and L. R. F. Carvalho, Quim. Nova, 1997, 20, 245–251 CAS.
- S. R. Souza, P. C. Vasconcellos and L. R. F. Carvalho, Atmos. Environ., 1999, 33, 2563–2574 CrossRef CAS.
- S. R. Souza and L. R. F. Carvalho, J. Braz. Chem. Soc., 2001, 12, 755–762 CAS.
- G. O. da Rocha, A. Franco, A. G. Allen and A. A. Cardosa, J. Geophys. Res., 2003, 108(D7), 4207 CrossRef.
- D. Grosjean, Environ. Sci. Technol., 1989, 23, 1506–1514 CrossRef CAS.
- O. Massambani and F. Andrade, Atmos. Environ., 1994, 19, 3165–3169 CrossRef.
- L. Lazutin, P. C. Bezerra, M. A. Fagnani, H. S. Pinto, I. M. Martin, E. L. P. da Silva, M. G. S. Mello, A. Turtelli, V. Zhavkov and J. Zullo, Atmos. Environ., 1996, 30, 2729–2738 CrossRef CAS.
- R. Guardani, C. A. O. Nascimento, M. L. G. Guardani, M. H. R. B. Martins and J. Romano, J. Air Waste Manage. Assoc., 1999, 49, 316–323 CAS.
- R. Guardani and C. A. O. Nascimento, Int. J. Environ. Pollution, 2004, 22, 441–459 Search PubMed.
- R. Guardani, J. L. Aguiar, C. A. O. Nascimento, C. I. V. Lacava and Y. Yanagi, J. Air Waste Manage. Assoc., 2003, 53, 553–559 CAS.
- C. A. Muramoto, C. F. F. Lopes and C. I. V. Lacava, Proceeding of the 96th Annual Conference and Exhibition, Air & Waste Manage. Assoc., Paper No. 69894, San Diego, California, 2003 Search PubMed.
- O. R. Sanchez-Ccoyllo, P. L. S. Dias, M. F. Andrade and S. R. Freitas, Meteorol. Atmos. Phys., 2006, 92, 83–93 CrossRef.
- A. C. C. L. Tresmondi and E. Tomaz, Int. J. Environ. Pollut., 2004, 22, 490–506 Search PubMed.
- C. Boian and V. W. J. H. Kirchhoff, Atmos. Environ., 2005, 39, 6140–6146 CrossRef CAS.
- M. H. R. B. Martins, R. Anazia, M. L. G. Guardani, C. I. V. Lacava, J. Romano and S. R. Silva, Int. J Environ. Pollut., 2004, 22, 430–440 Search PubMed.
- FEEMA, 2007 Relatório Anual de Qualidade do Ar, Rio de Janeiro, Brazil, 2008, http://www.inea.rj.gov.br/fma/Relatorio_2007.pdf Search PubMed.
- E. Grosjean, D. Grosjean, L. F. Woodhouse and Y.-J. Yang, Atmos. Environ., 2002, 36, 2405–2419 CrossRef CAS.
- D. Grosjean, J. Braz. Chem. Soc., 1997, 8, 433–442 CAS.
- W. P. L. Carter, K. R. Darnall, R. A. Graham, A. M. Winer and J. N. Pitts, J. Phys. Chem., 1979, 83, 2305–2311 CrossRef CAS.
- Y. Akutsu, F. Toyoda, K. Tomita, F. Yoshizawa, M. Tamura and T. Yoshida, Atmos. Environ., 1991, 25A, 1383–1389 CrossRef CAS.
- E. Grosjean, D. Grosjean, M. P. Fraser and G. R. Cass, Environ. Sci. Technol., 1996, 30, 2704–2714 CrossRef CAS.
- M. P. Fraser, D. Grosjean, E. Grosjean, R. A. Rasmussen and G. R. Cass, Environ. Sci. Technol., 1996, 30, 1731–1743 CrossRef CAS.
- W. P. L. Carter, J. Air Waste Manage. Assoc., 1994, 44, 881–899 CAS.
- P. A. P. Pereira, L. M. B. Santos, E. T. Sousa and J. B. de Andrade, J. Braz. Chem. Soc., 2004, 15, 646–651.
- E. Grosjean, R. A. Rasmussen and D. Grosjean, Atmos. Environ., 1998, 32, 3371–3379 CrossRef CAS.
- D. Grosjean, E. Grosjean, L. F. R. Moreira and R. A. Rasmussen, 94th Air & Waste Management Association Annual Meeting, Paper No. 560, Orlando, Florida, 2001 Search PubMed.
- D. S. Alvim, L. V. Gatti, J. P. Orlando, M. H. Santos and A. Yamazaki, 48th Congresso Brasileiro de Quimica, Rio de Janiero, Brazil, 2006, http://www.abq.org.br/cbq/2008/trabalhos/5/5-87-4517.htm Search PubMed.
- A. S. Pimentel and G. Arbilla, Quim. Nova, 1997, 20, 252–260 CAS.
- I. C. B. Campos, A. S. Pimentel, S. M. Correa and G. Arbilla, J. Braz. Chem. Soc., 1999, 10, 203–208 CAS.
- W. P. L. Carter, Atmos. Environ., 1990, 24A, 481–516 CrossRef CAS.
- B. J. Finlayson-Pitts and J. N. Pitts, Chemistry of the upper and lower atmosphere; Theory, experiments, and applications, Academic Press, San Diego, 2000 Search PubMed.
- G. Arbilla and K. M. P. G. de Oliveira, Quim. Nova, 1999, 22, 790–800 CAS.
- G. Arbilla, E. M. Martins, A. Moreira and L. F. R. Moreira, J. Braz. Chem. Soc., 2002, 13, 308–317 CAS.
- E. M. Martins and G. Arbilla, Atmos. Environ., 2003, 37, 1715–1722 CrossRef CAS.
- W. Jaeschke, A. Wolf and A. Reinecke, Trans. Ecology Environ., 1999, 36, 906–910 Search PubMed.
- E. D. de Freitas, L. D. Martins, P. L. S. Dias and M. F. Andrade, Atmos. Environ., 2005, 39, 6352–6361 CrossRef.
- M. F. Andrade, R. Y. Ynoue, R. Harley and A. H. Miguel, Int. J. Environ. Pollut., 2004, 22, 460–475 Search PubMed.
- R. McRae and R. Harley, CIT photochemical airshed model, Systems manual. Pasadena, CA. California Institute of Technology, 1992 Search PubMed.
- M. G. Vivanco and M. F. Andrade, Atmos. Environ., 2006, 40, 1189–1198 CrossRef CAS.
- O. R. Sanchez-Ccoyllo, R. Y. Ynoue, L. D. Martins and M. F. Andrade, Atmos. Environ., 2006, 40, S552–S562 CAS.
- O. R. Sanchez-Ccoyllo, L. D. Martins, R. Y. Ynoue and M. F. Andrade, Environ. Fluid Mech., 2007, 7, 95–119 Search PubMed.
- L. D. Martins and M. F. Andrade, Proc. 8 ICSHMO, Foz de Iguacu, Brazil, 2006, pp.139–147 Search PubMed.
- L. D. Martins and M. F. Andrade, Water, Air, Soil Pollut., 2008, 195, 201–213 CAS.
- L. D. Martins, P. C. Vasconcellos, L. R. F. Carvalho and M. F. Andrade, Rev. Brasil. Meteorol., 2006, 21, 190–200 Search PubMed.
- J. B. Dallarosa, E. C. Teixeira and R. C. M. Alves, Water, Air, Soil Pollut., 2007, 178, 385–399 CrossRef CAS.
- A. Faiz, S. Gautam and E. Burki, Sci. Total Environ., 1995, 169, 303–310 CrossRef CAS.
- M. L. Bell, D. L. Davis, N. Gouveia, V. H. Borja-Aburto and L. A. Cifuentes, Environ. Res., 2006, 100, 431–440 CrossRef CAS.
- M. F. Andrade, O. R. Sanchez-Ccoyllo and R. M. Miranda, Regional Workshop on Public Health and Climate Benefits of Air Quality Management, São Paulo, Brazil, 2004, http://www.epa.gov/ies/pdf/brazil/bra_fatimaandrade.pdf Search PubMed.
- J. S. Gaffney and N. A. Marley, Atmos. Environ., 2009, 43, 23–36 CrossRef CAS.
- A. H. Bravo, A. P. Sanchez, E. R. Rosa, T. C. Keener and L. Mingming, Clean Technol. Environ. Policy, 2006, 8, 174–181 Search PubMed.
- M. Z. Jacobson, Energy Environ. Sci., 2009, 2, 148–173 RSC.
| This journal is © The Royal Society of Chemistry 2009 |
