Remarkable impact of KBr pelletization on spin switching: probing Hofmann-type 3D spin-crossover frameworks by solid-state optical spectroscopy
Chinmoy
Das
a and
Pradip
Chakraborty
 *ab
*ab
aDepartment of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur-721302, India. E-mail: pradipc@chem.iitkgp.ac.in
bSchool of Nano Science and Technology, Indian Institute of Technology Kharagpur, Kharagpur-721302, India
First published on 25th November 2025
Abstract
KBr pelletization profoundly influences the thermal and photoinduced spin-crossover behavior in dehydrated [Fe1−xMx(pz)Pd(CN)4] in bulk, nanoparticles and polymer composite forms by inducing tensile strain, defects, dopant-rattling, and electrostatic perturbations that alter ΔE0HL and cooperativity. It enables hidden thermal switching, dopant dynamics, and quantum-tunneling-driven LIESST relaxation, revealing mechanical processing as a versatile tool to tune spin-state energetics and cooperativity.
Spin-crossover (SCO) compounds based on 3d4–3d7 transition metal ions have attracted great interest for applications in molecular switches, sensors, memory, and display devices.1,2 These materials exhibit reversible transitions between high-spin (HS) and low-spin (LS) states under external stimuli such as temperature, light, or pressure.3 Thermal SCO occurs near the characteristic transition temperature (T1/2), where HS and LS populations are equal. The transition is governed by the zero-point energy gap (ΔE0HL) between spin states and the degree of cooperativity among spin centers. Cooperativity arises from elastic interactions due to volume changes during switching and is mediated by short- and long-range forces, including van der Waals contacts, π–π stacking, and phonon coupling through the lattice.4 Magnetic interactions via spin–phonon coupling, spin–sublattice exchange (HS–HS, LS–LS, HS–LS), and superexchange, further modulate the energetic landscape during the transition.5,6
At low temperatures, photoexcitation triggers the light-induced excited spin-state trapping (LIESST) effect, converting LS (1A1) to metastable HS (5T2) states.7,8 The reverse-LIESST process optically restores the LS state,9 while the relaxation temperature, T(LIESST), marks the point at which the photoinduced HS state thermally decays.10,11 Among SCO systems, Fe(II)-based 3D Hofmann-type frameworks [Fe(pz)M′(CN)4] (pz = pyrazine; M′ = Ni2+, Pd2+, Pt2+) are particularly notable for sharp, reversible transitions near room temperature and strong cooperative effects.12,13 Recent work has extended these materials to the nanoscale, where reduced particle size typically stabilizes the HS state and modifies hysteresis width.14,15 However, detailed optical studies remain limited, despite growing evidence that crystallinity, morphology, and polymeric environments strongly influence SCO behavior.16–20
Here, we report the pronounced impact of KBr pelletization on the thermal and photoinduced spin-state switching of dehydrated [Fe1−xMx(pz)Pd(CN)4] compounds, with varying particle sizes (bulk vs. nanoparticles), compositions (M = Zn2+, Co2+, Ni2+; x ∼ 0.34, 0.46 and 0.38, respectively), and polymeric environments [poly(methyl methacrylate) (PMMA) and polyvinylpyrrolidone (PVP)]. The compounds dispersed in KBr pellets include: compound 1, bulk [Fe(pz)Pd(CN)4]; compound 2, [Fe(pz)Pd(CN)4] NPs; compound 3, [Fe0.66Zn0.34(pz)Pd(CN)4] NPs; compound 4 [Fe0.54Co0.46(pz)Pd(CN)4] NPs; compound 5 [Fe0.62Ni0.38(pz)Pd(CN)4] NPs; compound 6 [Fe(pz)Pd(CN)4]-PMMA; compound 7 [Fe(pz)Pd(CN)4]-PVP. These materials, along with their average particle sizes, are summarized in Table S1.
Using solid-state optical absorption spectroscopy on thin, transparent KBr pellets (image shown in Fig. S1), we investigate both thermal and light-driven transitions (see experimental section in the SI). Compared with our earlier powder-phase magnetic data,15 the KBr-embedded samples exhibit notable changes in T1/2, hysteresis width, and cooperativity, not due to applied pressure but arising from tensile stress, internal electrostatic interactions, and other mechanical effects induced during pellet formation. Interestingly, the observed trends oppose those typically seen under physical pressure, highlighting the complex and nontrivial influence of mechanical processing on spin-state energetics.
X-ray strain analysis21 (strain vs. sin2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ψ plots are shown in Fig. 1 and calculated stress values are summarized in Table S2) of compound 2 reveals positive slopes indicative of tensile stress. Both normal and shear components are tensile and significant, implying strong internal strain that stabilizes the HS state. Cross-sectional HRTEM (Fig. S3) of compound 2 confirms that nanoparticles embedded within KBr maintain their near-square morphology (average size ∼47 ± 13 nm) after pelletization and crystallinity, with distinct lattice fringes from both the nanoparticles and KBr. The crystalline ionic environment of KBr plays a pivotal role in tuning the spin-state energetics through elastic and electrostatic interactions.
ψ plots are shown in Fig. 1 and calculated stress values are summarized in Table S2) of compound 2 reveals positive slopes indicative of tensile stress. Both normal and shear components are tensile and significant, implying strong internal strain that stabilizes the HS state. Cross-sectional HRTEM (Fig. S3) of compound 2 confirms that nanoparticles embedded within KBr maintain their near-square morphology (average size ∼47 ± 13 nm) after pelletization and crystallinity, with distinct lattice fringes from both the nanoparticles and KBr. The crystalline ionic environment of KBr plays a pivotal role in tuning the spin-state energetics through elastic and electrostatic interactions.
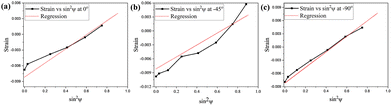 | ||
Fig. 1 Strain vs. sin2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ψ plot recorded on compound 2 at different ψ angles (a) 0°, (b) −45° and (c) −90°. ψ plot recorded on compound 2 at different ψ angles (a) 0°, (b) −45° and (c) −90°. | ||
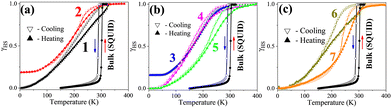
Fig. 2 shows the thermal spin-state switching of dehydrated compounds 1–7 in KBr pellets (Table S3), alongside magnetic data for bulk [Fe(pz)Pd(CN)4]. High-resolution, temperature-dependent absorption spectra (Fig. S4) reveal LS-specific 1A1 → 1MLCT transitions at 475–560 nm and HS-specific 5T2 → 5MLCT transitions at 440–515 nm. Spin-state changes are monitored by tracking absorbance at 525 nm, a maximum of the LS 1MLCT band (dashed arrow in Fig. S4). Upon cooling, the HS-related 5MLCT band weakens and redshifts, signaling HS depopulation, while the LS band intensifies and redshifts due to shorter Fe–ligand bonds (∼0.2 Å), which enhance orbital overlap and MLCT character.22 This spectral evolution enables determination of the normalized high-spin fraction (γHS) as a function of temperature (Fig. 2).23
Fig. 2a shows that compound 1 exhibits a complete but gradual transition with a 14 K hysteresis (T1/2↓ = 190 K, T1/2↑ = 204 K; Tavg1/2 = 197 K), compared to a sharp transition at 296 K in the bulk sample.13 The 99 K downshift (see Table S3) indicates significant HS stabilization due to pelletization-induced strain, inhomogeneous dispersion within the KBr matrix and lattice defects that lower ΔE0HL and alter cooperativity. Compound 2 (Fig. 2a) shows an incomplete, gradual transition near 193 K (Table S3) with a smaller hysteresis, reflecting further HS stabilization, reduced cooperativity, and electrostatic perturbations from the KBr environment.
For doped nanoparticles (compounds 3–5, Fig. 2b), KBr embedding further modifies SCO energetics. Compound 3 undergoes a gradual, incomplete transition near 176 K, while compound 4 displays a complete transition with reopened hysteresis (Tavg1/2 156 K, Table S3). Compound 5 shows a complete transition with Tavg1/2 = 214 K, consistent with LS stabilization upon Ni2+ substitution. In Zn(II)-, Co(II)-, and Ni(II)-doped nanoparticles (compounds 3–5), the dopant ionic radii closely match that of Fe(II) in the HS state [Zn(II), Co(II)] or LS state [Ni(II)], generating chemical pressure—negative for Zn(II)/Co(II), favoring HS stabilization, and positive for Ni(II), promoting LS stabilization. However, significant shifts in Tavg1/2 and cooperativity between KBr-pelletized and powder-phase samples indicate that factors beyond ionic size are at play. These include matrix confinement, defect-induced dopant dynamics, lattice fluctuations, and surface effects. Pelletization introduces structural defects, especially near dopant sites, enabling localized diffusion or anharmonic “dopant rattling”—a dynamics driven by size mismatch, dopant mobility, short mean-free paths, and low activation barriers. The extent of rattling is influenced by the rigidity of the doped framework and enhanced by the nanoparticle surface-to-volume ratio, impacting local lattice dynamics, spin-state energetics, and cooperativity. These processes modify elastic coupling among Fe(II)–Fe(II), Fe(II)–dopant, dopant–dopant, and dopant–matrix. Additionally, paramagnetic dopants introduce uncompensated magnetic exchange with Fe(II), further affecting cooperativity by influencing the elastic and magnetic coupling networks.
In polymer composites, compound 6 (PMMA-based, Fig. 2c) exhibits a complete transition with a 12 K hysteresis (Tavg1/2 = 183 K), downshifted by ∼95 K from the powder phase (Table S3).
PMMA's weakly interacting ester groups minimally affect ΔE0HL, while pelletization-induced strain, microcracks, and sample inhomogeneity broaden the energy distribution and stabilized the HS state. Despite these effects, the preserved hysteresis width suggests that key elastic and magnetic exchange interactions remain largely intact.
A particularly noteworthy case is compound 7 (PVP-based, Fig. 2c), which appears LS-locked in powder form15 but exhibits a distinct thermal transition upon KBr embedding (Tavg1/2 = 242 K, ΔT = 14 K). Coordination between PVP's pyrrolidone groups and Fe(II) stabilizes the LS state; however, mechanical stress and electrostatic heterogeneity (arising from cationic, anionic, and dipolar interactions) within the KBr matrix reduce ΔE0HL and modify the local crystal field, thereby enabling thermal access to the HS state, unlocking the SCO behavior while preserving cooperativity. This case exemplifies how mechanical and electrostatic perturbations can revive SCO behavior in an otherwise LS-stabilized polymer composite.
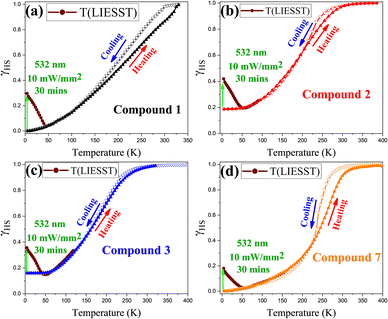
LIESST studies show partial HS photoconversion (20–30%) only in compounds 1–3 and 7 (Fig. 3a–d), with corresponding high-resolution T(LIESST) spectra presented in Fig. S5, whereas compounds 4–6 exhibit no detectable photoresponse and no observable spectral change (Fig. S6).
This is likely due to fast HS → LS relaxation or poor photoconversion efficiency. Pelletization-induced defects, internal strain, and electrostatic disorder strongly influence photodynamics, broadening ΔE0HL and producing either suppressed or quasi-steady-state LIESST responses. Elastic frustration or spin frustration from lattice imperfections and domain pinning further reduce efficiency. The similar photoresponses and T(LIESST) values for pelletized compounds 1–3 and 7 suggest comparable HS → LS relaxation dynamics. In contrast, near-complete LS → HS photoconversion has been reported for analogous nanoparticles on sapphire disks,17 underscoring the pronounced influence of the KBr matrix.
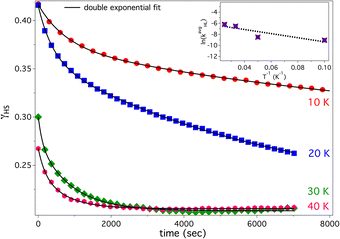
For isothermal relaxation, the sample is first irradiated at 4 K with a 532 nm laser (10 mW mm−2) to generate the metastable HS state, then rapidly heated (10 K min−1) to the relaxation temperature. Spectra are recorded at fixed intervals to monitor decay of the 1MLCT band at 525 nm (Fig. S7). Due to partial HS relaxation during rapid heating—especially at 30 and 40 K—initial γHS values vary. To account for this, spectra are collected every 5 min (10 K) and 3 min (20–40 K). Isothermal relaxation of compound 2 (Fig. 4) shows bi-exponential (stretched) decay kinetics with weak temperature dependence. This trend is reflected in the ln(kavgHL) vs. 1/T plot (Fig. 4 inset), with fitted curves closely matching the data. The average relaxation rate constant, kavgHL, calculated using earlier reported equation,24 is plotted against inverse temperature. The resulting relaxation behavior consistent with quantum tunneling through vibronically coupled states in an inhomogeneous lattice. The slow, non-Arrhenius behavior arises from strain and electrostatic disorder, which broadens the ΔE0HL distribution and induces elastic or spin frustration. Temperature-independent tunneling dominates at low temperatures, while varying residual γHS values (except at 30 and 40 K) reflect non-uniform ΔE0HL and local heterogeneity within the KBr matrix.
In summary, KBr pelletization profoundly alters the thermal and photoinduced SCO behavior of dehydrated [Fe1−xMx(pz)Pd(CN)4] frameworks by introducing tensile strain, defects, and electrostatic fields that reshape ΔE0HL and cooperative interactions. These effects stabilize the HS state, shift T1/2 downward, and can restore SCO activity in otherwise LS-locked systems. The LIESST response—characterized by partial photoconversion and quantum-tunneling-driven relaxation—further underscores the sensitivity of spin-state energetics to mechanical processing. Such effects are likely more pronounced in KBr pellets, as the process involves compressing 3D porous clathrates into a thin, quasi-2D form, thereby introducing additional structural constraints and strain within the material. This work establishes pelletization as a simple yet powerful route to tune SCO dynamics, uncover hidden transitions, and design responsive molecular materials with tailored optical and magnetic functionalities.
P. C. gratefully acknowledges financial support from the ANRF Core Research Grant (Grant No. CRG/2023/003032) and the DST-DAAD Project (Grant No. DST/INT/DAAD/P-18/2022). C. D. acknowledges the research fellowship provided by the Indian Institute of Technology Kharagpur. We sincerely thank the Central Research Facility (CRF), IIT Kharagpur, for providing access to the 2D XRD and HRTEM facilities. In particular, we express our gratitude to Dr Subhabrata Chakraborty and Mr Tapas Pal, CRF, IIT Kharagpur, for their valuable assistance in cross-sectional HRTEM sample preparation and measurements. We also acknowledge Mr Biswanath Hembram, Department of Civil Engineering, IIT Kharagpur, for his kind support and guidance in the stress analysis.
Conflicts of interest
All authors declare no conflicts of interest.Data availability
All data supporting the findings of this study are provided in the supplementary information (SI) and are also available from the corresponding author upon reasonable request. Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc06296a.Notes and references
- C. Bartual-Murgui, A. Akou, C. Thibault, G. Molnár, C. Vieu, L. Salmon and A. Bousseksou, J. Mater. Chem. C, 2015, 3, 1277–1285 RSC.
- C. Lefter, S. Rat, J. S. Costa, M. D. Manrique-Juárez, C. M. Quintero, L. Salmon, I. Séguy, T. Leichle, L. Nicu, P. Demont, A. Rotaru, G. Molnár and A. Bousseksou, Adv. Mater., 2016, 28, 7508–7514 CrossRef CAS.
- P. Gütlich and H. A. Goodwin, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Springer, Berlin, Heidelberg, 2004, pp. 1–47 Search PubMed.
- H. Spiering, in Spin Crossover in Transition Metal Compounds III, ed. P. Gütlich and H. A. Goodwin, Springer, Berlin, Heidelberg, 2004, pp. 171–195 Search PubMed.
- H. Banerjee, M. Kumar and T. Saha-Dasgupta, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 174433 CrossRef.
- S. Karmakar, P. Chakraborty and T. Saha-Dasgupta, J. Phys. Chem. C, 2024, 128, 16179–16188 CrossRef CAS.
- S. Decurtins, P. Gütlich, C. P. Köhler, H. Spiering and A. Hauser, Chem. Phys. Lett., 1984, 105, 1–4 CrossRef CAS.
- G. Li, O. Stefanczyk, K. Kumar, L. Guérin, K. Okuzono, K. Tran, M. Seydi Kilic, K. Nakabayashi, K. Imoto, A. Namai, Y. Nakamura, S. Ranjan Maity, F. Renz, G. Chastanet and S. Ohkoshi, Angew. Chem., Int. Ed., 2025, 64, e202423095 CrossRef CAS PubMed.
- A. Hauser, Chem. Phys. Lett., 1986, 124, 543–548 CrossRef CAS.
- J.-F. Létard, J. Mater. Chem., 2006, 16, 2550–2559 RSC.
- G. Chastanet, C. Desplanches, C. Baldé, P. Rosa, M. Marchivie and P. Guionneau, Chem. Sq., 2018, 2, 2 Search PubMed.
- V. Niel, J. M. Martinez-Agudo, M. C. Muñoz, A. B. Gaspar and J. A. Real, Inorg. Chem., 2001, 40, 3838–3839 CrossRef CAS.
- C. Das, S. Dey, A. Adak, C. Enachescu and P. Chakraborty, Cryst. Growth Des., 2023, 23, 3496–3508 CrossRef CAS.
- H. Peng, S. Tricard, G. Félix, G. Molnár, W. Nicolazzi, L. Salmon and A. Bousseksou, Angew. Chem., Int. Ed., 2014, 53, 10894–10898 CrossRef CAS PubMed.
- C. Das, A. Dutta, D. Coltuneac, L. Stoleriu and P. Chakraborty, Dalton Trans., 2025, 54, 7923–7940 RSC.
- N.-T. Yao, M.-J. Shang, H.-H. Lu, Q. Liu, W. Wen, S.-H. Zhang, L. Zhao, H. Oshio, Y.-S. Meng and T. Liu, Inorg. Chem. Front., 2024, 11, 3159–3167 RSC.
- T. Delgado, C. Enachescu, A. Tissot, A. Hauser, L. Guénée and C. Besnard, J. Mater. Chem. C, 2018, 6, 12698–12706 RSC.
- T. Delgado, A. Tissot, C. Besnard, L. Guénée, P. Pattison and A. Hauser, Chem. – Eur. J., 2015, 21, 3664–3670 CrossRef CAS.
- P. Chakraborty, M.-L. Boillot, A. Tissot and A. Hauser, Angew. Chem., Int. Ed., 2013, 52, 7139–7142 CrossRef CAS.
- V. Rubio-Giménez, C. Bartual-Murgui, M. Galbiati, A. Núñez-López, J. Castells-Gil, B. Quinard, P. Seneor, E. Otero, P. Ohresser, A. Cantarero, E. Coronado, J. A. Real, R. Mattana, S. Tatay and C. Martí-Gastaldo, Chem. Sci., 2019, 10, 4038–4047 RSC.
- I. C. Noyan and J. B. Cohen, Residual Stress, Springer, New York, NY, 1987 Search PubMed.
- I. Krivokapic, P. Chakraborty, R. Bronisz, C. Enachescu and A. Hauser, Angew. Chem., Int. Ed., 2010, 49, 8509–8512 CrossRef CAS.
- P. Chakraborty, C. Enachescu, C. Walder, R. Bronisz and A. Hauser, Inorg. Chem., 2012, 51, 9714–9722 CrossRef CAS PubMed.
- C. Enachescu, A. Hauser, J.-J. Girerd and M.-L. Boillot, ChemPhysChem, 2006, 7, 1127–1135 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |