Electrochemical upcycling biomass-derived methyl 2-furoate and CO2 into monomers for recyclable polyesters
Pengfei
Shi
ab,
Xinyu
Chai
c,
Yuefeng
Wang
b,
Chenbao
Lu
 *b,
Huiping
Ji
*d,
Yuezeng
Su
*a and
Xiaodong
Zhuang
*b,
Huiping
Ji
*d,
Yuezeng
Su
*a and
Xiaodong
Zhuang
 *b
*b
aSchool of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai, P. R. China
bThe Soft2D Lab, State Key Laboratory of Synergistic Chem-Bio Synthesis, State Key Laboratory of Metal Matrix Composites, Shanghai Key Laboratory of Electrical Insulation and Thermal Ageing, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: zhuang@sjtu.edu.cn
cSchool of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 201306, China
dKey Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, China Institute of Zhejiang University-Quzhou, Quzhou, China
First published on 24th November 2025
Abstract
We developed a site-selective electrochemical carboxylation strategy that converts biomass-derived methyl 2-furoate into dimethyl furan-2,5-dicarboxylate using CO2 as a sustainable C1 source. The reaction afforded the target monomer in 27% yield with excellent regioselectivity (>99%). Gram-scale synthesis, polymerization, and methanolysis recycling demonstrated efficient production and recyclability of furan-based polyesters.
The conversion of renewable biomass into high-value chemicals offers a sustainable pathway to address fossil resource depletion, environmental pollution, and energy security challenges.1,2 Among biomass-derived materials, furan-based polyesters, such as poly(ethylene 2,5-furandicarboxylate) (PEF), have attracted significant attention as a potential substitute for poly(ethylene terephthalate) (PET) owing to their superior thermal and mechanical stability.3 At the heart of this class of materials lies bio-based 2,5-furandicarboxylic acid (FDCA), a pivotal monomer serving an analogous role to purified terephthalic acid (PTA) in PET synthesis. The synthesis of FDCA currently relies largely on the oxidation pathway starting from 5-hydroxymethylfurfural (HMF). Nevertheless, the scalability of this pathway remains constrained by its reliance on edible C6 sugar feedstocks and the economically challenging production process of HMF.4 In contrast, furfural, a platform chemical readily obtained from D-xylose in xylan or hemicellulose,5 represents a more economical and sustainable feedstock. Therefore, developing an efficient process for the conversion of furfural to FDCA is of great importance for enhancing biomass valorization and promoting a sustainable circular bioeconomy.
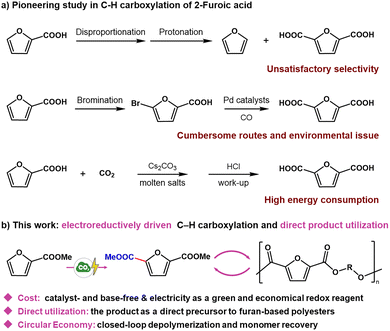
Current approaches for converting biomass-derived furfural to FDCA primarily rely on 2-furoic acid as a key intermediate. Among them, the disproportionation of 2-furoic acid is one of the earliest methods, but it generates significant byproducts that complicate purification.6 A more recent four-step carbonylation sequence affords a 65% overall yield, yet the lengthy procedure and associated waste generation remain major drawbacks.7 In 2016, Banerjee et al.2 reported a direct Cs2CO3/CO2 carboxylation of 2-furoic acid with 89% yield; however, this method requires substantial energy input (Fig. 1a). Electrosynthesis has recently emerged as a prominent strategy for enabling challenging organic transformations.8–11 By exploiting electricity as a controllable redox equivalent, this technique enables the generation of highly reactive radical intermediates under mild conditions,12–14 offering a promising route for direct C–H carboxylation of 2-furoic acid. Despite this potential, research on electrochemical strategies for the direct conversion of furfural derivatives remains very limited.
 | ||
| Fig. 1 (a) Pioneering studies on the C–H carboxylation of 2-furoic acid. (b) Electrochemical C–H carboxylation with CO2. | ||
In this work, an electroreduction-mediated C–H carboxylation strategy was developed using methyl 2-furoate (MFA), a furfural-derived substrate, to synthesize dimethyl furan-2,5-dicarboxylate (dm-FDCA) (Fig. 1b). Under the optimized conditions, the reaction proceeded with excellent regioselectivity (>99%) and afforded dm-FDCA in a yield of 27%. The obtained dm-FDCA was subsequently polymerized with ethylene glycol and isosorbide to yield the corresponding furan-based polyesters, PEF and poly(isosorbide 2,5-furanoate) (PIF), respectively. Notably, these polymers were chemically recyclable via methanolysis, enabling the recovery of dm-FDCA in yields of 32% from PEF and 25% from PIF. This work demonstrates a sustainable and integrated CO2-biomass valorization platform featuring: (a) direct generation of a polymer-grade monomer, enabling immediate conversion into furan-based polyesters; (b) a low cost, base- and catalyst-free electrosynthetic protocol; (c) electricity as energy input with no pollution generation; and (d) recyclability enabled by facile depolymerization.
The electrosynthesis was carried out in a membrane-less cell, MFA was employed as a model substrate, and an organic solution containing a suitable supporting electrolyte salt was used as the electrolyte (Fig. S1). Upon completion of the constant-current bulk electrolysis, the electrolyte was collected and the product was analysed by gas chromatography-mass spectrometry (GC-MS) (Fig. S2–S8) and nuclear magnetic resonance (NMR) spectroscopy (Fig. S9–S12) for structural confirmation. To systematically investigate the effects of electrode materials, electrolyte types, and solvents on reaction selectivity and conversion, parallel experiments were conducted under 1 atm CO2 at a constant current of 20 mA. The role of the anode was first examined. The results showed that using a cheap and reusable graphite felt (GF) as the anode, in CH3CN with tetrabutylammonium iodide (nBu4NI) as the electrolyte, enabled smooth formation of the target product 1 (Table 1, entry 1). In contrast, replacing the anode with Pt led to a dramatic decrease in MFA conversion, and no target product was detected (Table 1, entry 2). When Mg or Zn sacrificial anodes were employed, the reaction pathway shifted significantly, with the byproduct 2 becoming the major product (25% and 22% yield, respectively) (Table 1, entries 3 and 4), highlighting the decisive role of the anode material in controlling reaction selectivity. Further investigation of the cathode effect revealed that using GF for both the anode and cathode afforded the highest yield of 27%, with complete product selectivity and exclusive C5 regioselectivity (Table 1, entry 5), emphasizing the importance of synergistic interactions between the electrodes.
| Entry | Variations from optimal conditions | Time (h) | Conversion (%) | Yield (%) | |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| a Reaction conditions: undivided cell, GF as the anode and cathode, constant current = 20 mA, MFA (0.8 mmol), CO2 atmosphere in balloon (1 atm), nBu4NI (0.8 mmol, 1.0 equiv) in CH3CN (8.0 mL), room temperature, 3.5 h. CH3CN = acetonitrile, DMF = N,N-dimethylformamide, NMP = N-methyl-2-pyrrolidone, DMSO = dimethyl sulfoxide. NR = no reaction. | |||||
| 1 | (−) Pt|GF (+) | 3.5 | 100 | 21 | 5 |
| 2 | (−) Pt|Pt (+) | >10 | 5 | Trace | Trace |
| 3 | (−) Pt|Mg (+) | 3.5 | 100 | 8 | 25 |
| 4 | (−) Pt|Zn (+) | 3.5 | 100 | 7 | 22 |
| 5 | (−) GF|GF (+) | 3.5 | 99 | 27 | Trace |
| 6 | n Bu4N•Br | 3.5 | 100 | 15 | Trace |
| 7 | n Bu4N•BF4 | 3.5 | 97 | 5 | Trace |
| 8 | n Bu4N•PF6 | 3.5 | 99 | 2 | Trace |
| 9 | n Bu4N•CIO4 | 3.5 | 95 | 3 | Trace |
| 10 | Et4N·I | 3.5 | 95 | 23 | Trace |
| 11 | DMF | 3.5 | 98 | 20 | Trace |
| 12 | NMP | 3.5 | 99 | 22 | Trace |
| 13 | DMSO | 3.5 | 99 | 12 | Trace |
| 14 | w/o electricity or CO2 | 10 | NR | NR | NR |
The electrolyte effect was subsequently investigated using the optimal electrode configuration (Table 1, entries 6–10). When nBu4NBr was employed (entry 6), the reaction afforded a moderate yield of product 1 (15%), indicating that bromide is less effective than iodide. In contrast, when non-halide electrolytes (entries 7–9) such as nBu4NBF4, nBu4NPF6, and nBu4NClO4 were used, the yield dropped sharply to 5%, 2%, and 3%, respectively, confirming the essential role of halide anions in promoting the reaction. Using Et4NI (entry 10) restored the yield to 23%, further verifying that the iodide anion (I−) is significantly more effective than Br− owing to its lower oxidation potential and superior ability to generate reactive iodine species. The effect of different solvents was also evaluated. Among these, CH3CN emerged as the optimal medium for this transformation (Table 1, entries 11–13). Finally, control experiments verified the essential roles of both electricity and CO2; in the absence of either, no product was detected (Table 1, entry 14).
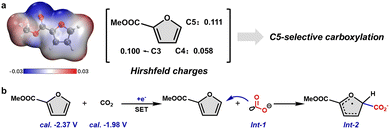
Density functional theory (DFT) calculations were performed to elucidate the reaction mechanism. We first sought to clarify the origin of the exclusive C5 regioselectivity observed in the reaction. The electrostatic potential map (Fig. 2a, left) revealed distinct positive character at the C3, C4, and C5 positions of MFA, indicating that these sites are electrophilically activated and susceptible to nucleophilic attack. To quantitatively assess the relative reactivity of these sites, condensed Fukui functions for nucleophilic attack (fA+) were computed using Hirshfeld population analysis.15 The computed fA+ values (Fig. 2a, right) align well with the experimental selectivity, with the C5 position exhibiting the highest value (0.111) compared to C3 (0.100) and C4 (0.058), clearly rationalizing the observed C5 regioselectivity (Table S1). Furthermore, the preferred reaction pathway was investigated by comparing the relative reduction potentials of the substrate MFA and CO2, and the species with the more positive reduction potential is thermodynamically favored to undergo prior reduction.16 The calculated half-wave potentials (vs. SCE in CH3CN) are −2.37 V for MFA and −1.98 V for CO2 (Table S2). Since CO2 possesses a substantially more positive reduction potential than MFA, it undergoes preferential single-electron reduction to form the CO2 radical anion (Int-1). This radical intermediate then attacks the C5 position of MFA, leading to the formation of intermediate Int-2 (Fig. 2b).
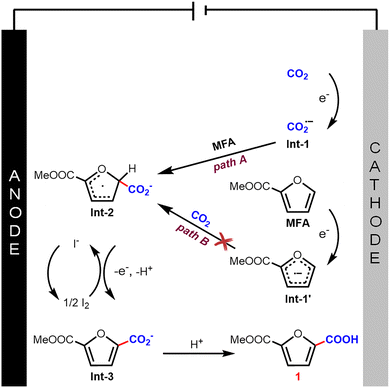
Based on the mechanistic insights from DFT calculation results and previous reports,9,10 a plausible reaction mechanism is depicted in Fig. 3. The reaction is initiated by the single-electron reduction of CO2 (path A), leading to the formation of the CO2 radical anion (Int-1), rather than proceeding via the alternative pathway involving initial reduction of MFA to form Int-1′ (path B). The resulting Int-1 then undergoes regioselective nucleophilic attack at the C5 position of MFA to yield intermediate Int-2. Subsequent oxidation of Int-2 by iodine, accompanied by proton loss, delivers intermediate Int-3, while I- is reoxidized to I2 at the anode. Finally, protonation of Int-3 affords the desired carboxylic acid product 1. The formation of product 2 follows a parallel pathway through intermediates Int-1 and Int-2. In contrast, when a sacrificial anode is used, the anodic oxidation of I− to I2 is suppressed. Consequently, Int-2 cannot be oxidized by iodine and instead undergoes a second single-electron reduction at the cathode. This reduction generates an α-carbanion intermediate Int-4 adjacent to the ester group. The rapid decarboxylation of the β-carboxylate ester renders Int-4 unreactive toward CO2,17 leading instead to protonation and formation of product 2 (Fig. S13).
To further highlight the practical applicability of this methodology, we carried out a gram-scale reaction. The process was scaled up by a factor of twenty, employing 16 mmol (2.02 g) of MFA as the substrate. Remarkably, under the optimized conditions, the carboxylation proceeded efficiently and could be seamlessly coupled with the subsequent methylation step without the need for intermediate purification. This streamlined transformation furnished dm-FDCA in an isolated yield of 22% (648.2 mg) (Fig. S14). Then, the obtained dm-FDCA was polymerized with two structurally contrasting biomass-derived diols: the highly flexible ethylene glycol (R1) and rigid, asymmetric isosorbide (R2), obtaining the polyesters PEF and PIF (Fig. 4a). Both polyesters were synthesized via a typical two-step melt polycondensation method. Gel permeation chromatography (GPC) analysis revealed narrow molecular weight distributions for both polyesters (PDI = 1.01 for PEF and 1.04 for PIF), with PIF showing a slightly higher average molecular weight (Mn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599 g mol−1, Mw = 18
599 g mol−1, Mw = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 g mol−1) compared to that of PEF (Mn = 16
361 g mol−1) compared to that of PEF (Mn = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159 g mol−1, Mw = 16
159 g mol−1, Mw = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421 g mol−1) (Fig. S15, S16 and Table S3). The XRD patterns clearly reveal the significant crystallization differences between PEF and PIF (Fig. 4b). PEF exhibited multiple sharp diffraction peaks, characteristic of a highly ordered crystalline structure. In contrast, PIF displayed only a broad amorphous halo with no discernible sharp reflections, demonstrating its predominantly amorphous state. Thermogravimetric analysis (TGA) shows distinct thermal decomposition behaviours for the two polymers (Fig. 4c). PEF underwent a single-stage decomposition process within the range of 320–425 °C, whereas PIF displayed a typical two-stage decomposition, occurring at 235–340 °C and 340–445 °C, respectively (Fig. S17), which could be attributed to the presence of thermally labile groups in the isosorbide moiety. Differential scanning calorimetry (DSC) was employed to investigate the thermal transition behaviours of the polyesters during heating and cooling cycles (Fig. 4d and Table S4). PIF exhibited a substantially higher glass transition temperature (Tg = 90 °C) compared to PEF (Tg = 78 °C), highlighting the enhanced chain rigidity imparted by the isosorbide unit. This contrast extends to their crystallization and melting behaviours.
421 g mol−1) (Fig. S15, S16 and Table S3). The XRD patterns clearly reveal the significant crystallization differences between PEF and PIF (Fig. 4b). PEF exhibited multiple sharp diffraction peaks, characteristic of a highly ordered crystalline structure. In contrast, PIF displayed only a broad amorphous halo with no discernible sharp reflections, demonstrating its predominantly amorphous state. Thermogravimetric analysis (TGA) shows distinct thermal decomposition behaviours for the two polymers (Fig. 4c). PEF underwent a single-stage decomposition process within the range of 320–425 °C, whereas PIF displayed a typical two-stage decomposition, occurring at 235–340 °C and 340–445 °C, respectively (Fig. S17), which could be attributed to the presence of thermally labile groups in the isosorbide moiety. Differential scanning calorimetry (DSC) was employed to investigate the thermal transition behaviours of the polyesters during heating and cooling cycles (Fig. 4d and Table S4). PIF exhibited a substantially higher glass transition temperature (Tg = 90 °C) compared to PEF (Tg = 78 °C), highlighting the enhanced chain rigidity imparted by the isosorbide unit. This contrast extends to their crystallization and melting behaviours.
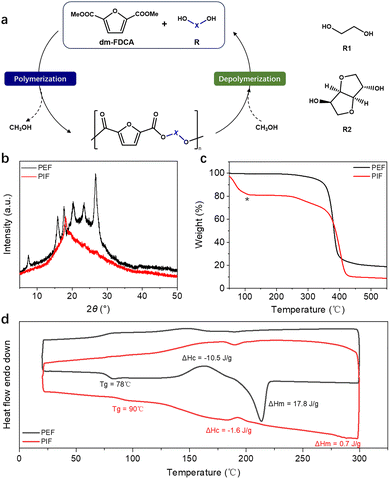 | ||
| Fig. 4 Closed-loop recyclability and material properties of PEF and PIF. (a) Illustration of the closed-loop process, (b) XRD patterns, (c) TGA curves, and (d) DSC analysis. Note: the significant mass loss below 150 °C of PIF (*) in Fig. 4c is attributed to the evaporation of residual solvent. | ||
In parallel, the chemical recycling potential of PEF and PIF was preliminarily investigated, given that efficient recycling strategies are essential for realizing a sustainable, circular polymer economy. Specifically, a mixture of the polymer, methanol (as the depolymerization reagent, 70 equivalents relative to the polymer repeating unit), and Zn(OAc)2 (1.0 mol% relative to the polymer repeating unit) was refluxed at 90 °C for 5 hours. The resulting depolymerization products were directly identified by GC-MS, and the dm-FDCA content was quantified using an external standard method (Fig. S18). Quantitative analysis revealed depolymerization yields of 32% for PEF and 25% for PIF under identical conditions. These results provide an important proof of concept for the chemical recyclability of both polyesters. Such preliminary findings demonstrate the potential of furan-based polyesters not only as sustainable materials but also as candidates for closed-loop recycling within a circular plastic economy.
To analyze the industrial potential of the major synthetic routes reported and the electrochemical carboxylation pathway developed herein, we constructed a Multi-Criteria Decision Analysis (MCDA) framework (Table S5). The comparison shows that the electrochemical route possesses a unique combination of advantages, including ambient operation, catalyst- and base-free conditions, low waste generation, direct CO2 fixation, safe and scalable process, and compatibility with renewable electricity. The protocol has been successfully verified on a gram-scale, and further yield improvement would position this strategy as a highly promising candidate for large-scale production.
In summary, we have developed a direct and site-selective electrochemical strategy for synthesizing high-value furandicarboxylic acid derivatives from the biomass-derived platform molecule MFA, using CO2 as an economical and sustainable carboxylation source. Employing GF as both the cathode and anode, the reaction afforded dm-FDCA in 27% yield under mild, catalyst- and base-free conditions, and its feasibility was further demonstrated on a gram scale. Melt polymerization with ethylene glycol and isosorbide produced PEF and PIF with moderate molecular weights and narrow dispersity (PDI < 1.1), while methanolysis enabled recovery of dm-FDCA in 32% and 25% yields, respectively. This work establishes a sustainable and integrated CO2-biomass valorization platform based on a base- and catalyst-free electrosynthetic protocol driven by electricity as a clean redox reagent. The process directly furnishes a polymer-grade monomer for furan-based polyesters and offers inherent recyclability via facile depolymerization.
This work was financially supported by the National Natural Science Foundation of China (NSFC: 52573229 and 22473098) and NSFC Young Scientists Fund (22208210 and 22208213). The authors also thank the support from Science and Technology Commission of Shanghai Municipality (25ZR1401213 and 25DZ3001002) and China Postdoctoral Science Fund (2025M770964). The computations in this paper were run on the π 2.0 cluster supported by the Center for High Performance Computing at Shanghai Jiao Tong University (SJTU). We thank the support from the Instrumental Analysis Center of SJTU.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc05972k.Notes and references
- J. Q. Bond, D. M. Alonso, D. Wang, R. M. West and J. A. Dumesic, Science, 2010, 327, 1110–1114 CrossRef CAS PubMed.
- A. Banerjee, G. R. Dick, T. Yoshino and M. W. Kanan, Nature, 2016, 531, 215–219 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- S. Zhao, M. Cheng, J. Li, J. Tian and X. Wang, Chem. Commun., 2011, 47, 2176–2178 RSC.
- T. Pan, J. Deng, Q. Xu, Y. Zuo, Q. X. Guo and Y. Fu, ChemSusChem, 2013, 6, 47–50 CrossRef CAS PubMed.
- S. Zhang, G. Shen, Y. Deng, Y. Lei, J.-W. Xue, Z. Chen and G. Yin, ACS Sustainable Chem. Eng., 2018, 6, 13192–13198 CrossRef CAS.
- G.-Q. Sun, P. Yu, W. Zhang, W. Zhang, Y. Wang, L.-L. Liao, Z. Zhang, L. Li, Z. Lu and D.-G. Yu, Nature, 2023, 615, 67–72 CrossRef CAS.
- Y. You, W. Kanna, H. Takano, H. Hayashi, S. Maeda and T. Mita, J. Am. Chem. Soc., 2022, 144, 3685–3695 CrossRef CAS.
- Z. Zhao, Y. Liu, S. Wang, S. Tang, D. Ma, Z. Zhu, C. Guo and Y. Qiu, Angew. Chem., Int. Ed., 2023, 62, e202214710 Search PubMed.
- T. von Münchow, S. Dana, Y. Xu, B. Yuan and L. Ackermann, Science, 2023, 379, 1036–1042 CrossRef.
- C. M. Hendy, G. C. Smith, Z. Xu, T. Lian and N. T. Jui, J. Am. Chem. Soc., 2021, 143, 8987–8992 CrossRef CAS PubMed.
- M. C. Leech and K. Lam, Nat. Rev. Chem., 2022, 6, 275–286 CrossRef PubMed.
- C. Kingston, M. D. Palkowitz, Y. Takahira, J. C. Vantourout, B. K. Peters, Y. Kawamata and P. S. Baran, Acc. Chem. Res., 2020, 53, 72–83 CrossRef CAS.
- Y. Ma, J. Liang, D. Zhao, Y.-L. Chen, J. Shen and B. Xiong, RSC Adv., 2014, 4, 17262–17264 Search PubMed.
- Y. You, W. Kanna, H. Takano, H. Hayashi, S. Maeda and T. Mita, J. Am. Chem. Soc., 2022, 144, 3685–3695 CrossRef CAS.
- V. K. Rawat, H. Hayashi, H. Katsuyama, S. R. Mangaonkar and T. Mita, Org. Lett., 2023, 25, 4231–4235 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |