Anion-mediated regulation of open metal sites in metal–organic framework materials or high-performance solid-state electrolytes
Pucheng
Zhao
 ,
Yanyan
Xu
,
Mingjie
Liu
,
Tengfei
Liu
,
Junling
Xu
,
Lianyi
Shao
,
Yanyan
Xu
,
Mingjie
Liu
,
Tengfei
Liu
,
Junling
Xu
,
Lianyi
Shao
 ,
Xiaoyan
Shi
* and
Zhipeng
Sun
,
Xiaoyan
Shi
* and
Zhipeng
Sun
 *
*
School of Materials and Energy, Guangdong University of Technology, Guangzhou, Guangdong, P. R. China. E-mail: shixy222@gdut.edu.cn; zpsunxj@gdut.edu.cn
First published on 24th November 2025
Abstract
In this study, a Cu-MOF was successfully synthesized, and the coordination behavior between its open metal sites (OMSs) and various anions was systematically investigated. It was determined that the NO3− anion exhibits the most effective coordination with the Cu2+ sites. This optimal interaction endows the resulting LiNO3@Cu-MOF with superior electrochemical properties: an ionic conductivity of 1.19 × 10−3 S cm−1, a lithium-ion transference number of 0.69, and stable lithium plating/stripping for 1600 hours at 0.2 mA cm−2. This work elucidates the profound role of OMSs in regulating anion coordination and provides a novel design strategy for high-performance, MOF-based solid-state electrolytes.
The urgent need for clean energy exploration and development has heightened the importance of advanced energy storage devices.1 Currently, lithium-ion batteries (LIBs) are the most widely used and commercialized technology in this field.2 However, their extensive application has revealed considerable safety concerns.3 A primary issue involves liquid electrolytes, which can promote lithium dendrite growth. These dendrites can penetrate the separator, leading to internal short circuits.4 This failure mechanism presents serious risks such as electrolyte leakage, flammability, and potential toxicity. To mitigate these hazards, the development of solid-state electrolytes (SSEs) is critically imperative.5 SSEs offer significant advantages, including enhanced safety, higher energy density, and longer cycle life. Nevertheless, they present their own challenges: polymer-based SSEs suffer from low ionic conductivity and high interfacial impedance; oxide-based SSEs exhibit high grain boundary resistance and brittleness; and sulfide-based SSEs are hampered by poor environmental stability and severe interfacial side reactions.6 To overcome these limitations, composite solid-state electrolytes (CSEs), which synergistically combine the properties of different materials, have attracted significant attention due to their potential for improving ionic conductivity, mechanical strength, and interfacial stability.7 Alternatively, the persistent development of novel SSE varieties remains a critical and ongoing research direction.
Metal–organic frameworks (MOFs) are crystalline porous materials with ultra-high surface areas and tunable structures, formed by coordinating metal clusters with organic ligands. These properties make MOFs promising for SSEs, where they can enhance ion transport and interfacial stability, either as standalone components or as fillers in polymer composites.8 Anionic MOFs, characterized by their negatively charged frameworks, are a promising platform for developing high-performance SSEs. Their inherent negative charge provides continuous hopping sites for charge carriers, which lowers the activation energy for ion transport and significantly enhances the cation transference number.9 The synthesis of anionic MOFs typically employs one of three strategies: (i) introducing anionic segments onto organic ligands, (ii) using inherently anionic ligands, or (iii) post-synthetically decorating anionic coordinating ligands onto the open metal sites (OMSs) of pre-formed MOFs.9,10 Among these, the third strategy offers superior flexibility, as the concentration and type of coordinating ligand can be precisely modulated to tune the MOF's charge properties and optimize lithium-ion transport kinetics.
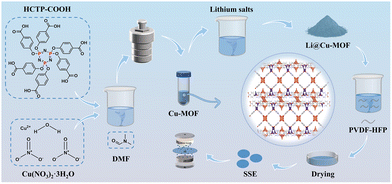
This study synthesized a copper-based metal–organic framework (Cu-MOF) rich in open metal sites (OMSs) by reacting highly purified hexa(4-carboxyphenoxy)cyclotriphosphazene (HCTP-COOH) with copper nitrate (Fig. 1 and Fig. S1). Using a post-synthetic modification strategy, the Cu-MOF was subsequently reacted with lithium salts with different anions (NO3−, SO42−, Cl−, Br−, C2H3O2−) to produce anionic Li@Cu-MOFs.11 Initially, the OMSs are weakly coordinated to dimethylformamide (DMF) solvent molecules. These solvent molecules can be substituted by anions during the modification process, leading to the formation of anionic Li@Cu-MOFs. Because different anions have varying coordination strengths, the coordination environment of the copper metal centers can be modulated. This modulation, in turn, adjusts the thermal stability and lithium-ion transport properties of Li@Cu-MOFs. As the results indicated, the NO3− anion most effectively modifies the coordination environment around the copper centers, primarily shifting the coordination geometry from four to five. This specific modification yielded the highest Li+ transference number (tLi+ = 0.69) and ionic conductivity (σ = 1.19 × 10−3 S cm−1), alongside exceptional rate performance and stable lithium plating/stripping behavior. This strategy presents a new approach for modifying MOF-based SSEs and may contribute to the development of safe, long-lifespan energy storage systems.12
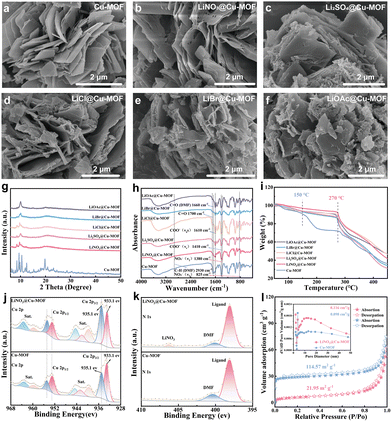
As shown in the scanning electron microscopy (SEM) images (Fig. 2a), the pristine Cu-MOF exhibits a block-like morphology with a relatively uniform, thick structure. Upon reaction with various lithium salts, the Cu-MOF underwent corrosion to varying degrees. Among these, the LiNO3@Cu-MOF retained the most intact structure (Fig. 2b–f). Corresponding X-ray diffraction (XRD) analysis confirms the successful synthesis of the target Cu-MOF, as evidenced by the close match between the experimental and simulated patterns (Fig. 2g and Fig. S2a).13 Furthermore, the absence of CuO diffraction peaks, even after water exposure, confirms the material's aqueous stability (Fig. S2b and c).14 While the XRD patterns of the anionic Li@Cu-MOF series are consistent with the pristine framework—indicating structural preservation—a general reduction in crystallinity is observed, with LiNO3@Cu-MOF maintaining the highest crystallinity among the series.
The bonding characteristics of these anionic MOFs were investigated by Fourier transform infrared (FT-IR) spectroscopy (Fig. 2h). The presence of characteristic absorption peaks at approximately 1610 cm−1 and 1410 cm−1, corresponding to the coordinated carboxyl groups, confirms the structural integrity of the organic linker in all frameworks. In the pristine Cu-MOF spectrum, the apparent peaks at approximately 2930 cm−1 and 1660 cm−1 are attributed to the C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of DMF, respectively, indicating that the OMSs were originally occupied by solvent molecules after synthesis. Following anionic modification, the significant reduction in intensity of these peaks confirms the replacement of most DMF molecules. In the spectra of Li2SO4@Cu-MOF, LiCl@Cu-MOF, LiBr@Cu-MOF, and LiOAc@Cu-MOF, a new absorption peak appears at approximately 1700 cm−1. This peak is attributed to the C
O bonds of DMF, respectively, indicating that the OMSs were originally occupied by solvent molecules after synthesis. Following anionic modification, the significant reduction in intensity of these peaks confirms the replacement of most DMF molecules. In the spectra of Li2SO4@Cu-MOF, LiCl@Cu-MOF, LiBr@Cu-MOF, and LiOAc@Cu-MOF, a new absorption peak appears at approximately 1700 cm−1. This peak is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of free, uncoordinated carboxyl groups, suggesting partial framework hydrolysis occurred in these samples. This phenomenon is absent in both the pristine Cu-MOF and LiNO3@Cu-MOF, further demonstrating the superior stability of the NO3−-modified framework. Furthermore, two new absorption peaks observed at approximately 1380 cm−1 and 825 cm−1 correspond to the asymmetric stretching vibration (ν3) and out-of-plane bending vibration (ν2) of NO3−, respectively, confirming the compatibility of LiNO3 when modifying the MOFs.15
O stretching vibration of free, uncoordinated carboxyl groups, suggesting partial framework hydrolysis occurred in these samples. This phenomenon is absent in both the pristine Cu-MOF and LiNO3@Cu-MOF, further demonstrating the superior stability of the NO3−-modified framework. Furthermore, two new absorption peaks observed at approximately 1380 cm−1 and 825 cm−1 correspond to the asymmetric stretching vibration (ν3) and out-of-plane bending vibration (ν2) of NO3−, respectively, confirming the compatibility of LiNO3 when modifying the MOFs.15
Thermogravimetric analysis (TGA) was employed to evaluate the thermal stability of the pristine and anionic modified MOFs. As shown in Fig. 2i, the pristine Cu-MOF exhibited a substantial weight loss of 25% between 30–250 °C, whereas the LiNO3@Cu-MOF showed a significantly lower loss of only 6.5% over the same temperature range. The rapid decomposition of the pristine Cu-MOF above 150 °C can be attributed to the loss of abundant volatile solvent molecules (DMF). In contrast, the LiNO3@Cu-MOF remained stable until approximately 270 °C, which further confirms the replacement of coordinated DMF molecules with NO3− anions. Furthermore, although the other anionic MOFs (Li2SO4@Cu-MOF, LiCl@Cu-MOF, LiBr@Cu-MOF, and LiOAc@Cu-MOF) also exhibited improved thermal stability compared to the pristine framework, their stability was inferior to that of LiNO3@Cu-MOF, consistent with the partial hydrolysis observed in their structures.
The coordination environments of the Cu centres in the pristine and anionic-modified Li@Cu-MOFs were characterized by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 2j and Fig. S3, the introduction of anionic ligands alters the Cu2+ coordination environment. This is evidenced by a change in the relative intensities of the two Cu 2p3/2 peaks at approximately 933.1 eV and 935.1 eV, corresponding to four- and five-coordinate Cu species, respectively. The spectral changes indicate a shift from a predominantly four-coordinate geometry toward a five-coordinate one. The extent of this shift varies among the anionic Li@Cu-MOFs (Fig. S3 and Table S1), reflecting their differing coordination capabilities. Based on the peak area ratio, LiNO3@Cu-MOF exhibits the most pronounced modification. The superior coordination of the nitrate anion is attributed to its compact, monodentate binding mode, which allows it to effectively occupy the axial coordination sites on Cu2+ with minimal steric demand and sufficient bond strength. In contrast, the sulfate anion is hindered by the spatial requirements of its multidentate coordination, while chloride and bromide anions, being weak-field ligands with larger ionic radii, exhibit significantly weaker competitive coordination. The successful incorporation of NO3− is further confirmed by the N 1s spectrum of LiNO3@Cu-MOF, which shows a characteristic peak at a binding energy of 406.5 eV. The superior coordinating ability of NO3− suggests its potential for optimizing the electrochemical performance of these MOFs as SSEs.16–18 To further elucidate the structural consequences of anionic modification, the textural properties of the pristine and modified Cu-MOF were quantitatively assessed using N2 physisorption (Fig. 2l). The analysis reveals a profound alteration in porosity following the incorporation of LiNO3. The pristine Cu-MOF exhibits a type IV isotherm, characteristic of a mesoporous structure, with a high specific surface area of 114.57 m2 g−1 and a total pore volume of 0.116 cm3 g−1. In contrast, the LiNO3@Cu-MOF sample shows a drastic reduction in its textural parameters. Its specific surface area plummets to 21.95 m2 g−1—a decrease of approximately 80%—while the corresponding pore volume is reduced to 0.098 cm3 g−1. This remarkable diminution in both surface area and pore volume provides compelling, quantitative evidence for the successful and extensive incorporation of LiNO3 within the MOF matrix. The branched anions and lithium cations occupy a substantial portion of the internal pore space. This effective pore blockage not only confirms the success of the post-synthetic modification but also suggests a high loading density of the anionic modifier, which is consistent with the strong coordinating ability of the nitrate anion as established by XPS analysis.
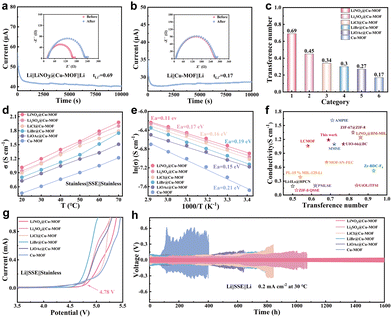
The lithium-ion transport properties of the anionic MOF-based SSEs were systematically evaluated. The lithium transference number (tLi+) was determined using Li||SSE||Li symmetric cells. As shown in Fig. 3a–c and Fig. S4, the calculated tLi+ values for the anionic modified MOFs were 0.69, 0.45, 0.34, 0.30, and 0.27, respectively, all of which are superior to that of the pristine Cu-MOF (0.17). Furthermore, the ionic conductivity was investigated by electrochemical impedance spectroscopy (EIS) from 20 to 70 °C (Fig. 3d and Fig. S5). At 30 °C, the pristine Cu-MOF exhibited an ionic conductivity of 6.095 × 10−4 S cm−1. In contrast, the anionic-modified variants—LiNO3@Cu-MOF, Li2SO4@Cu-MOF, LiCl@Cu-MOF, LiBr@Cu-MOF, and LiOAc@Cu-MOF—showed enhanced conductivities of 1.197 × 10−3, 1.060 × 10−3, 1.092 × 10−3, 9.643 × 10−4, and 8.831 × 10−4 S cm−1, respectively (Table S2). The corresponding activation energies for ion migration, derived from Arrhenius plots (Fig. 3e), were 0.11, 0.17, 0.16, 0.19, 0.15, and 0.21 eV. Collectively, these results confirm that LiNO3@Cu-MOF possesses the most favorable combination of a high lithium transference number, high ionic conductivity, and low activation energy, indicating highly efficient lithium-ion transport. This superior performance is attributed to the compact, monodentate coordination of NO3− anions, which anchor firmly to the Cu2+ open metal sites. This binding creates a fixed anionic framework that provides well-defined hopping sites for rapid Li+ migration while simultaneously enhancing the structural stability of the MOF.
The electrochemical stability of the synthesized SSEs was further investigated by linear sweep voltammetry (LSV). As shown in Fig. 3g, the LiNO3@Cu-MOF-based SSE exhibits a superior electrochemical stability window of 4.78 V, notably wider than those of the other modified counterparts. This enhanced stability is attributed to the coordination of NO3− anions with the Cu2+ centres, which stabilizes the MOF framework and suppresses oxidative decomposition at high potentials, thereby ensuring compatibility with high-voltage cathodes. Furthermore, long-term cycling stability was evaluated in Li||SSE||Li symmetric cells. The cell with the LiNO3@Cu-MOF SSE demonstrated exceptional stability, maintaining a stable lithium stripping/plating process for over 1600 hours at 0.2 mA cm−2 with a consistently low overpotential of ∼30 mV (Fig. 3h). In stark contrast, the cell with the pristine Cu-MOF SSE failed after only 400 hours, while cells with other anionic MOFs exhibited severe polarization. This remarkable performance is ascribed to the abundant incorporation of LiNO3, which enables: (1) strong nitrate coordination that guides uniform lithium deposition and suppresses dendrite growth, and (2) the creation of favorable ion-conduction pathways for rapid Li+ transport. The synergy between this stabilized interface and efficient bulk ion conduction underpins the superior performance.
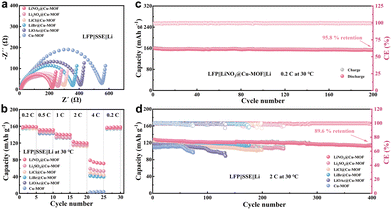
The performance of the anionic MOF-based SSEs was further evaluated in practical full cells with a LiFePO4 (LFP) cathode. EIS confirmed that the LFP||LiNO3@Cu-MOF||Li cell exhibited the smallest charge-transfer resistance (Rct) at room temperature (Fig. 4a), consistent with its high ionic conductivity and low activation energy. This favorable kinetics translated to superior rate performance (Fig. 4b), with the cell delivering specific capacities of 159.04, 150.93, 140.10, 120.36, and 72.97 mAh g−1 at 0.2, 0.5, 1, 2, and 4C, respectively. The well-maintained voltage profiles across these current densities further attest to its excellent rate capability (Fig. S6a). The long-term cycling performance further highlights the material's advantage. At 0.2C, the LiNO3@Cu-MOF-based cell delivered an initial discharge capacity of 159.69 mAh g−1 with a Coulombic efficiency of 99.29%, retaining 153.02 mAh g−1 (95.82% retention) after 200 cycles with minimal polarization (Fig. 4c and Fig. S6b). This robustness extended to a high rate of 2C (Fig. 4d and Fig. S6c), where the cell exhibited an initial capacity of 126.91 mAh g−1 (99.88% CE) and retained 113.7 mAh g−1 (89.6%) after 400 cycles. In stark contrast, the control cell with the pristine Cu-MOF SSE suffered rapid capacity decay, failing after 55 cycles and delivering only 96.48 mAh g−1 by the 74th cycle.
To elucidate this performance disparity, the morphology of the cycled SSE membranes was investigated. The pristine LiNO3@Cu-MOF membrane exhibited a thickness consistent with literature values (Fig. S7a and b), a smooth surface, and good flexibility (Fig. S7c and d). The membrane also demonstrated exceptional thermal stability, retaining its structural integrity and flexibility without cracking or melting across a temperature range of 60 to 180 °C (Fig. S7e–h). Following 200 cycles at 0.2C, the LiNO3@Cu-MOF cell maintained a uniform electrode surface and a smooth, dendrite-free lithium anode (Fig. S7i–l). This stable interfacial morphology, in sharp contrast to the severe dendrite formation observed with the unmodified Cu-MOF, is attributed to the enhanced ionic conductivity and Li+ transference number of LiNO3@Cu-MOF. These optimized transport properties originate from the anionic mediation of its open metal sites. Post-cycling XRD analysis further confirmed the structural integrity of both materials, with patterns showing consistent peak positions and intensities, indicating no degradation of the crystalline frameworks (Fig. S8). The pronounced contrast underscores the critical role of LiNO3 modification in ensuring long-term electrochemical and structural stability. Furthermore, the LiNO3@Cu-MOF composite also demonstrated excellent flame retardancy (Fig. S9), a property attributed to its cyclotriphosphazene-based ligand and stable architecture. This inherent safety supports sustained battery performance, underscoring its potential for safer solid-state batteries.
In summary, a crystalline Cu-MOF with abundant open metal sites (OMSs) was hydrothermally synthesized from a HCTP-COOH ligand. These OMSs were leveraged to anchor various anions (NO3−, SO42−, Cl−, Br−, CH3COO−), regulating the coordination geometry of Cu2+ centres to form a series of anionic Li@Cu-MOFs. The NO3− anion proved most effective, yielding a LiNO3@Cu-MOF solid-state electrolyte (SSE) with exceptional properties: an ionic conductivity of 1.19 × 10−3 S cm−1 and a Li+ transference number of 0.69 at 30 °C. This SSE enabled stable lithium stripping/plating for over 1600 hours with a minimal overpotential of 30 mV (0.2 mA cm−2) and, in full cells with a LiFePO4 cathode, delivered outstanding cycling stability (95.82% capacity retention after 200 cycles at 0.2C and 89.6% after 400 cycles at 2C). This work elucidates the critical role of OMSs in anion coordination and provides a novel design strategy for high-performance, MOF-based solid-state electrolytes.
Pucheng Zhao: investigation, experiment, writing – original draft, writing-review and data curation. Yanyan Xu: validation and data curation. Mingjie Liu: investigation and validation. Tengfei Liu: verification and summary. Lianyi Shao: visualization, Junling Xu: visualization. Xiaoyan Shi: writing – review and data curation, funding acquisition, validation, project administration, and supervision. Zhipeng Sun: validation, resources, project administration, supervision, and funding acquisition.
Thanks to the financial support from the National Natural Science Foundation of China (Grant No. 22271066), Guangzhou Science and Technology Plan Project (No. 2024A03J0087), Guangdong Association for Science and Technology (No. SKXRC2025380), and the analytical support from the School of Materials and Energy, Guangdong University of Technology.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the findings of this study are available within the article and its supplementary information (SI). The supplementary information includes experimental details, electrochemical characterization data, supplementary figures and tables, and references. See DOI: https://doi.org/10.1039/d5cc06129f.Notes and references
- S. Duan, L. Qian, Y. Zheng, Y. Zhu, X. Liu, L. Dong, W. Yan and J. Zhang, Adv. Mater., 2024, 32, 202314120 Search PubMed.
- Y. Kim, C. Li, J. Huang, Y. Yuan, Y. Tian and W. Zhang, Adv. Mater., 2024, 36, 202407761 Search PubMed.
- Y. Ma, J. Wan, Y. Yang, Y. Ye, X. Xiao, D. T. Boyle, W. Burke, Z. Huang, H. Chen, Y. Cui, Z. Yu, S. T. Oyakhire and Y. Cui, Adv. Energy Mater., 2022, 12, 202103720 Search PubMed.
- X. Nie, Z. Chen, B. Deng, L. Shao, J. Xu, X. Shi and Z. Sun, Adv. Energy Mater., 2025, 15, 202500069 Search PubMed.
- X. Huang, T. Li, W. Fan, R. Xiao and X. B. Cheng, Adv. Energy Mater., 2024, 14, 202303850 Search PubMed.
- Q. Zeng, J. Wang, X. Li, Y. Ouyang, W. He, D. Li, S. Guo, Y. Xiao, H. Deng, W. Gong, Q. Zhang and S. Huang, ACS Energy Lett., 2021, 6, 2434–2441 CrossRef CAS.
- M. S. Nafis, Z. Liang, S. Lee and C. Ban, Nano Energy, 2024, 113, 110447 Search PubMed.
- H. He, N. Deng, X. Wang, L. Gao, C. Tang, E. Wu, J. Ren, X. Yang, N. Feng, D. Gao and X. Zhuang, Adv. Funct. Mater., 2025, 35, 2421670 CrossRef CAS.
- Q. Xia, K. Han, X. Ma, P. Qiu, Z. Li and X. Chen, Chem. Sci., 2024, 15, 17579–17589 RSC.
- F. Tao, L. Tian, Z. Liu, R. Cui, M. Liu, X. Kang and Z. Liu, J. Mater. Chem. A, 2022, 10, 14020–14027 RSC.
- M. Liu, Z. Chen, B. Chen, T. Liu, P. Zhao, J. Xu, L. Shao, X. Shi and Z. Sun, Chem. Commun., 2025, 61, 5637–5640 RSC.
- S. Ma, L. Shen, Q. Liu, W. Shi, C. Zhang, F. Liu, J. A. Baucom, D. Zhang, H. Yue, H. B. Wu and Y. Lu, ACS Appl. Mater. Interfaces, 2020, 12, 43824–43832 CrossRef CAS.
- Y. Ling, C. Song, Y. Feng, M. Zhang and Y. He, CrystEngComm, 2015, 17, 6314–6319 RSC.
- A. A. Alhazime, J. Inorg. Organomet. Polym., 2020, 30, 4459–4467 CrossRef CAS.
- Y. Liu, H. Zhang, R. Bai, H. Wang, Y. Zhao, Y. Zhou and X. Zhao, Chem. Eng. J., 2024, 485, 149876 CrossRef CAS.
- M. W. S. Chong, S. P. Argent, F. Moreau, W. J. F. Trenholme, C. G. Morris, W. Lewis, T. L. Easun and M. Schröder, Chem. – Eur. J., 2022, 28, e202201188 CrossRef CAS.
- P. J. Griffin, M. J. Dake, A. D. Remolina and L. Olshansky, Dalton Trans., 2023, 52, 8376–8383 RSC.
- S. Debnath, S. Laxmi, O. McCubbin Stepanic, S. Y. Quek, M. van Gastel, S. DeBeer, T. Krämer and J. England, J. Am. Chem. Soc., 2024, 146, 23704–23716 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |