Nicotine-derived ammonium salts as highly efficient catalysts for chemical fixation of carbon dioxide into cyclic carbonates under solvent-free conditions†
Abdol R. Hajipour*ab,
Yasaman Heidaria and
Gholamreza Kozehgaryc
aPharmaceutical Research Laboratory, Department of Chemistry, Isfahan University of Technology, Isfahan 84156, Islamic Republic of Iran. E-mail: haji@cc.iut.ac.ir; Fax: +98 313 391 2350; Tel: +98 313 391 3262
bDepartment of Neuroscience, University of Wisconsin, Medical School, 1300 University Avenue, Madison, 53706-1532, WI, USA
cDepartment of Chemistry, Faculty of Science, Shahid Beheshti University, G. C., Evin, Tehran 1983963113, Iran
First published on 1st July 2015
Abstract
A series of easily prepared nicotine-derived ammonium salts were applied for the first time as recyclable and efficient catalysts for the coupling of carbon dioxide and epoxides to form cyclic carbonates at low pressure without using additional organic solvents and co-catalysts. Remarkably, excellent yields and selectivity were achieved when 1-benzyl-1-methyl-2-pyridin-3-yl-pyrrolidinium bromide [MBNT]Br was used as the catalyst. Furthermore, the catalyst can be easily recovered and reused without a significant loss of activity. The influences of the catalyst structure and various reaction parameters on the catalytic activity were also investigated in detail.
Introduction
Carbon dioxide is an attractive C1 feedstock gas in organic synthesis as it is extremely practical, inexpensive, abundant, non-toxic, and non-flammable. As petroleum treasuries are exhausted, the development of efficient catalytic systems for CO2 fixation is consequently a significant and challenging theme.1–3 In this regard, the synthesis of cyclic carbonates from epoxides and CO2 has attracted widespread attention owing to the 100% atom efficiency of the reaction and the wide range of applications of cyclic carbonates such as polar aprotic solvents in organic and polymeric synthesis, electrolyte components for lithium batteries and intermediates in the syntheses of pharmaceuticals.4–6In the past few decades, a wide variety of homogeneous and heterogeneous catalysts have been developed for this reaction, such as alkali metal salts,7,8 metal oxides,9 Cs-loaded zeolite and alumina,10 transition metal salen complexes,11 zeolite,12 quaternary ammonium and phosphonium salts,13–18 and various ionic liquids (ILs).19–23 However, most of these catalysts suffer from one or more of the following drawbacks: low catalytic activity, the need for higher CO2 pressure and/or longer reaction times, the presence of expensive transition metal additives, and water or air sensitivity. Hence, the design of easily prepared, metal-free catalysts with enhanced catalytic activity for the conversion of CO2 to cyclic carbonate still remains a challenge.
In this study, a series of ammonium salts derived from nicotine (Scheme 1) are considered for the first time as catalysts towards the cycloaddition reaction of carbon dioxide with epoxide. These salts have advantages such as the facile preparation of various structures24,25 from nicotine and benzyl or alkyl halides, gratifying thermal behaviour and air/water stability. More importantly, Suzuki et al.26 reported the solubility of nicotine in carbon dioxide, and also that the tertiary nitrogen in the cation has the potential to react with CO2 to form the carbamate species, assumed to be an activated form of CO2.27 Indeed, the ammonium salt [MBNT]Br displayed high catalytic activity for conversion of CO2 and epoxide to cyclic carbonate and a respectable yield together with excellent selectivity was obtained without utilization of any organic solvents or additives.
Results and discussion
During the initial study, a series of ammonium salts based on nicotine have been applied to catalyse the cycloaddition reaction of styrene oxide (SO) and CO2. The results are summarized in Table 1. It can be seen that nicotine which contains basic active sites afforded styrene carbonate (SC) in 40% yield (Table 1, entry 1), while after quaternization, monobenzylnicotinum salts showed higher catalytic activities (Table 1, entries 2 and 3), indicating that without any anions the presence of only nicotine could not afford highly active centers for this reaction. Hence, the effects of halogen anions such as Cl− and Br− on the catalytic activity were examined, and it was found that [MBNT]Br showed a much higher activity than [MBNT]Cl (entry 3 vs. 2) which is probably ascribed to the nucleophilicity and leaving ability of the anion in the corresponding ammonium salt.28–30| Entry | Catalyst | Catalyst (mol%) | Yieldb (%) | Selectivitya (%) |
|---|---|---|---|---|
| a Reaction conditions: SO (10 mmol), CO2 (5 atm), 24 h, 120 °C, solventless.b Determined by GC using an internal standard technique. | ||||
| 1 | Nicotine | 3 | 40 | 81 |
| 2 | [MBNT]Cl | 3 | 86 | 93 |
| 3 | [MBNT]Br | 3 | 98 | 99 |
| 4 | [DBNT]Br | 3 | 2 | 3 |
| 5 | [MBNT]Br | 5 | 98 | 99 |
| 6 | [MBNT]Br | 2 | 81 | 91 |
To obtain more information on the effects of the tertiary amine group in the cation part on the reaction, dibenzylnicotinum bromide [DBNT]Br which has no basic active sites was investigated. [DBNT]Br was found to be inactive probably due to the loss of basic active sites and consequently the loss of its CO2 activation effect27 (Table 1, entry 4). This result indicated that the effect of the tertiary amine group was a significant factor in promoting the reaction. Therefore, [MBNT]Br was selected as the benchmark structure for further studies on the reaction parameters.
Subsequently, the effects of the catalyst loading on the SC synthesis were investigated. It can be observed that the SC yield decreased from 98% to around 81% with the catalyst loading decreasing from 3 to 2 mol% (Table 1, entry 6), while the SC yield remained almost constant when the catalyst loading was increased to 5 mol% (Table 1, entry 5). As a consequence, 3 mol% was chosen as the most suitable catalyst loading.
As shown in Fig. 1, the temperature had a pronounced positive influence on the cycloaddition reaction when it was varied from 80 to 100 °C. The SC yield was increased from about 71% to above 98% correspondingly, whereas it remained almost unchanged in the temperature range from 100 to 120 °C. Conclusively, 100 °C could be the optimal temperature for SC synthesis.
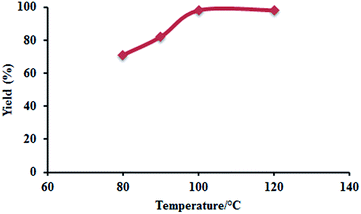 | ||
| Fig. 1 Influence of temperature on SC yield. Reaction conditions: SO (10 mmol), [MBNT]Br (3 mol%), CO2 (5 atm), 24 h. | ||
Commonly, a significant issue connected with utilizing CO2 as a reaction medium is the potential hazards of working at high pressure. Fig. 2 shows the effect of CO2 pressure on the yield of SC for the [MBNT]Br catalyst at 100 °C for 24 h. As is easily seen, the SC yield significantly increased from 79% to 98% when the initial CO2 pressure increased from 1 to 4 atm. Such a positive effect of CO2 pressure on the synthesis of cyclic carbonates from CO2 and epoxides has been reported in earlier studies.31–33 However, a further increase in pressure did not yield more reaction products. Therefore, 4 atm was considered as the optimal pressure for the reaction.
 | ||
| Fig. 2 Influence of the CO2 pressure on the yield of SC. Reaction conditions: SO (10 mmol), cat. amount (3 mol%), 100 °C, 24 h. | ||
The dependence of the SC yield on the reaction time was also evaluated. It was observed that the reaction time had a remarkable effect on the reaction. As shown in Fig. 3, the reaction proceeded rapidly within the first 4 h, and an almost quantitative yield (98%) could be achieved, while the reaction rate remained almost invariant after 4 h. Therefore, a reaction time of 4 h was appropriate for the synthesis of cyclic carbonates in this study.
 | ||
| Fig. 3 Influence of the reaction time on the yield of SC for [MBNT]Br (3 mol%), at 100 °C and a CO2 pressure of 4 atm. | ||
Experiments were performed to test the reusability of the [MBNT]Br catalyst under the optimum conditions. The catalyst was recovered after separation of the product from the reaction mixture by distillation under reduced pressure and then used for the next run under identical conditions.27e,34 As shown in Fig. 4, there was no significant drop in the SC yield after four runs, which indicated a high stability and reusability of the catalyst.
 | ||
| Fig. 4 The reusability results of the [MBNT]Br catalyst. Reaction condition: SO (10 mmol), catalyst (3 mol%), 100 °C, 4 h, CO2 (4 atm). | ||
Under the optimal reaction conditions, a series of epoxides were explored for cyclic carbonate synthesis in the presence of [MBNT]Br and the results are depicted in Table 2. The catalyst was found to be applicable to various terminal epoxides to provide the corresponding cyclic carbonates in high yields with excellent selectivity (up to 98%), while internal cyclohexene oxide exhibits the lowest activity (Table 2, entry 4) even at an elevated pressure and with a prolonged reaction time (Table 2, entry 5) probably due to steric hindrance as also illustrated by other authors.35–37
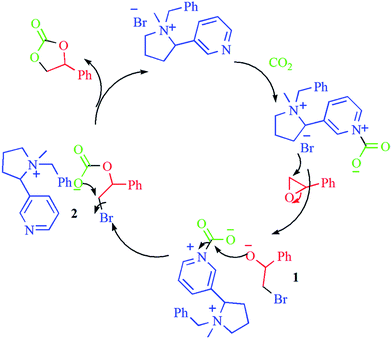
Based on previous reports38–40 and the obtained results, we proposed a plausible mechanism for the coupling of epoxides with CO2 using [MBNT](Br) as a catalyst, as shown in Scheme 2. Firstly, the tertiary nitrogen atom coordinates reversibly with CO2 to afford the carbamate salt,41,42 which could be an activated form of CO2. In parallel, the nucleophilic attack of the bromide anion on the less sterically hindered carbon atom of the epoxide produces the ring-opened intermediate 1. Then, nucleophilic attack of the intermediate 1 on the carbamate salt furnishes the alkyl carbonate anion 2. Finally, subsequent intramolecular ring-closure would form the cyclic carbonate and regenerate the [MBNT](Br) catalyst.
Conclusions
Ammonium salts based on nicotine proved to be highly efficient and recyclable catalysts for the synthesis of cyclic carbonates from epoxides and CO2 without utilizing any co-catalyst and co-solvent. It was found that monobenzylnicotinum salts showed much better catalytic performances, which could activate CO2 through the tertiary nitrogen in the cation part. In addition, the catalyst can be recovered and reused four times without any significant loss of its initial activity.Experimental
General
Commercial reagents were purchased from Merck Company and used without further purification. CO2 of a purity of 99.99% was commercially available. 1H NMR spectra were recorded on Bruker 250 and 400 spectrometers using TMS as an internal standard in CDCl3 or DMSO. Elemental analyses were performed by using a Leco, CHNS-932 elemental analyzer. Mass spectra were recorded on a Shimadzu QP 1100 BX Mass Spectrometer and FT-IR spectra were obtained using KBr pellets on a JASCO 680-Plus spectrophotometer. Melting points were measured with Gallenkamp melting apparatus. Thermal analysis comprising TG and DSC of the samples was performed using a STA system, model 409 PC Luxx under a flow of nitrogen at a rate of heating of 10 °C min−1 up to 600 °C. Gas chromatography (GC) (BEIFIN 3420 gas chromatograph equipped with a flame ionization detector and a Varian CP SIL 5CB column: 30 m, 0.32 mm, 0.25 mm) was used for consideration of reaction conversions and yields.Catalysts preparation and characterization
General procedure for the cycloaddition reaction
All the cycloadditions were conducted in a 100 mL stainless-steel reactor equipped with a magnetic stirrer under a CO2 atmosphere and the reactor was put into a bath of 100 °C and then pressurized to the appropriate pressure with CO2. In the typical procedure, [MBNT]Br (3 mol%), 1,2-dichlorobenzene (internal standard of GC) and 10 mmol of styrene oxide (SO) were added into the reactor. Then, the atmosphere inside the reactor was replaced with CO2 and the pressure was adjusted to 4 atm at 100 °C and the stirrer was started. After 4 h, the reactor was cooled to room temperature, and the excess of CO2 was gradually released, and the mixture was evaporated under vacuum. The residuals were purified using silica gel column chromatography (eluent: ethyl acetate) to give the corresponding cyclic carbonate. The cycloaddition products are known compounds and were characterized by comparing their IR, ESI-mass, 1H and 13C NMR spectra with those found in the literature.43−45Acknowledgements
We gratefully acknowledge the funding support received for this project from the Isfahan University of Technology (IUT), IR Iran (A. R. H.) and Grant GM 33138 (A. E. R.) from the National Institutes of Health, USA. Further financial support from the Center of Excellence in Sensor and Green Chemistry Research (IUT) is gratefully acknowledged.Notes and references
- D. J. Darensbourg, Chem. Rev., 2007, 107, 2388 CrossRef CAS PubMed
.
- L. Gu and Y. Zhang, J. Am. Chem. Soc., 2010, 132, 914 CrossRef CAS PubMed
.
- S. Klaus, M. W. Lehenmeier, E. Herdtweck, P. Deglmann, A. K. Ott and B. Rieger, J. Am. Chem. Soc., 2011, 133, 13151 CrossRef CAS PubMed
.
-
(a) A. Baba, H. Kashiwagi and H. Matsuda, Organometallics, 1987, 6, 137 CrossRef CAS
; (b) A. Baba, H. Kashiwagi and H. Matsuda, Tetrahedron Lett., 1985, 26, 1323 CrossRef CAS
.
- T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS PubMed
.
- D. J. Darensbourg, S. J. Lewis, J. L. Rodgers and J. C. Yarbrough, Inorg. Chem., 2003, 42, 581 CrossRef CAS
.
- J. W. Huang and M. Shi, J. Org. Chem., 2003, 68, 6705 CrossRef CAS PubMed
.
- N. Kihare, N. Hara and T. Endo, J. Org. Chem., 1993, 58, 6198 CrossRef
.
- K. Yamaguchi, K. Ebitani, T. Yoshida, H. Yoshida and K. Kaneda, J. Am. Chem. Soc., 1999, 121, 4526 CrossRef CAS
.
- M. Tu and R. J. Davis, J. Catal., 2001, 199, 85 CrossRef CAS
.
- J. Chun, S. Kang, N. Kang, S. M. Lee, H. J. Kim and S. U. Son, J. Mater. Chem. A, 2013, 1, 5517 CAS
.
- R. Srivastava, D. Srinivas and P. Ratnasamy, Appl. Catal., A, 2005, 289, 128 CrossRef CAS PubMed
.
- V. Caló, A. Nacci, A. Monopoli and A. Fanizzi, Org. Lett., 2002, 4, 2561 CrossRef PubMed
.
- K. Motokura, S. Itagaki, Y. Iwasawa, A. Miyaji and T. Baba, Green Chem., 2009, 11, 1876 RSC
.
- J.-Q. Wang, K. Dong, W.-G. Cheng, J. Sun and S.-J. Zhang, Catal. Sci. Technol., 2012, 2, 1480 CAS
.
- J. Tharun, Y. Hwang, R. Roshan, S. Ahn, A. C. Kathalikkattil and D.-W. Park, Catal. Sci. Technol., 2012, 2, 1674 CAS
.
- Y. Tsutsumi, K. Yamakawa, M. Yoshida, T. Ema and T. Sakai, Org. Lett., 2010, 12, 5728 CrossRef CAS PubMed
.
- L.-N. He, H. Yasuda and T. Sakakura, Green Chem., 2003, 5, 92 RSC
.
- J. Tharun, A. C. Kathalikkattil, R. Roshan, D.-H. Kang, H.-C. Woo and D.-W. Park, Catal. Commun., 2014, 54, 31 CrossRef CAS PubMed
.
- M. Liu, F. Wang, L. Shi, L. Liang and J. Sun, RSC Adv., 2015, 5, 14277 RSC
.
- S. Zhang, Y. Chen, F. Li, X. Lu, W. Dai and R. Mori, Catal. Today, 2006, 115, 61 CrossRef CAS PubMed
.
- Y. Zhang and J. Y. G. Chan, Energy Environ. Sci., 2010, 3, 408 CAS
.
- M. B-Sogo, H. Garcia and C. Aprile, Catal. Sci. Technol., 2015, 5, 1222 Search PubMed
.
- O. Lanitou, D. Dimotikali, E. Yannakopoulou and K. Papadopoulos, Chem. Eng. J., 2007, 134, 72 CrossRef CAS PubMed
.
- A. R. Hajipour and F. Karimi, Synth. Commun., 2010, 40, 1784 CrossRef CAS PubMed
.
- J. Suzuki, Y. Yonei and C. Yokoyama, Kagaku Kogaku Ronbunshu, 1995, 21, 608 CrossRef CAS
.
-
(a) T. Endo and D. Nagai, Macromolecules, 2004, 37, 2007 CrossRef CAS
; (b) F. S. Pereira and E. R. deAzevedo, Tetrahedron, 2008, 64, 10097 CrossRef CAS PubMed
; (c) A. Wykes and S. L. MacNeil, Synlett, 2007, 107 CAS
; (d) M. Yoshizawa-Fujita and K. Johansson, Tetrahedron Lett., 2006, 47, 2755 CrossRef CAS PubMed
; (e) Z.-Z. Yang, L.-N. He, S.-Y. Peng and A.-H. Liu, Green Chem., 2010, 12, 1850 RSC
.
- L. N. Han, H. J. Choi, S. J. Choi, B. Y. Liu and D. W. Park, Green Chem., 2011, 13, 1023 RSC
.
- Y. Zhao, J. S. Tian, X. H. Qi, Z. N. Han, Y. Y. Zhuang and L. N. He, J. Mol. Catal. A: Chem., 2007, 271, 284 CrossRef CAS PubMed
.
- J. Sun, W. G. Cheng, W. Fan, Y. H. Wang, Z. Y. Meng and S. J. Zhang, Catal. Today, 2009, 148, 361 CrossRef CAS PubMed
.
- B. M. Bhanage, S. Fujita, Y. Ikushima and M. Arai, Appl. Catal., A, 2001, 219, 259 CrossRef CAS
.
- B. M. Bhanage, S. Fujita, Y. Ikushima, K. Torii and M. Arai, Green Chem., 2003, 5, 71 RSC
.
- J. Sun, S. I. Fujita, F. Zhao and M. Arai, Appl. Catal., A, 2005, 287, 221 CrossRef CAS PubMed
.
- The procedure for catalyst recycling: the catalyst was recovered after separation of the product from the reaction mixture through distillation and [MBNT]Br left in the bottom of the round flask was cooled and washed with ethyl acetate, then reused for the next run without further purification under identical conditions.
- Y. X. Zhou, S. Q. Hu, X. M. Ma, S. G. Liang, T. Jiang and B. X. Han, J. Mol. Catal. A: Chem., 2008, 284, 52 CrossRef CAS PubMed
.
-
(a) T. Iwasaki, N. Kihara and T. Endo, Bull. Chem. Soc. Jpn., 2000, 73, 713 CrossRef CAS
; (b) S. Brunaure, L. S. Deming, W. E. Deming and E. Teller, J. Am. Chem. Soc., 1940, 62, 1723 CrossRef
.
- L. Han, H.-J. Choi, S.-J. Choi, B. Liu and D.-W. Park, Green Chem., 2011, 13, 1023 RSC
.
- A. Barbarini and G. Sartori, Tetrahedron Lett., 2003, 44, 2931 CrossRef CAS
.
- J. Sun and S. J. Zhang, Tetrahedron Lett., 2008, 49, 3588 CrossRef CAS PubMed
.
- M. North and R. Pasquale, Angew. Chem., 2009, 121, 2990 CrossRef PubMed
.
- Z. Z. Yang, L. N. He, C. X. Miao and S. Chanfreau, Adv. Synth. Catal., 2010, 352, 2233–2240 CrossRef CAS PubMed
.
- M. North and R. Pasquale, Angew. Chem., Int. Ed., 2009, 48, 2946 CrossRef CAS PubMed
.
- J. Meléndez, M. North and R. Pasquale, Eur. J. Inorg. Chem., 2007, 3323 CrossRef PubMed
.
- J. Meléndez, M. North and P. Villuendas, Chem. Commun., 2009, 2577 RSC
.
- J.-L. Wang, J.-Q. Wang, L.-N. He, X.-Y. Dou and F. Wu, Green Chem., 2008, 10, 1218 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08488a |
| This journal is © The Royal Society of Chemistry 2015 |