Preparation of silver nanoparticles supported mesoporous silica microspheres with perpendicularly aligned mesopore channels and their antibacterial activities
Ren-Shu Huanga,
Bao-Fei Houb,
Hai-Tao Lib,
Xu-Cheng Fu*b and
Cheng-Gen Xieb
aChemistry and Life Science Department, West Anhui University, Lu'an, Anhui 237015, PR China
bAnhui Provincial Laboratory of Biomimetic Sensor and Detecting Technology, West Anhui University, Lu'an, Anhui 237015, PR China. E-mail: fxc8307@wxc.edu.cn; Fax: +86 5643305660; Tel: +86 5643305690
First published on 9th July 2015
Abstract
In this study, a facile and effective route for the preparation of silver nanoparticles supported surface mesoporous silica microspheres with perpendicularly aligned mesopore channels and their antibacterial activities were reported. The surface mesoporous silica microspheres (mSiO2) were synthesized by a sol–gel method. The mSiO2 were then functionalized with 3-aminopropyltriethoxysilane (APTS) to provide amino functional groups for the absorption of Ag+. Silver nanoparticles were directly created on the surface of mSiO2 by in situ chemical reduction of the Ag precursor using an ultrasonic wave reaction method. The prepared silver nanoparticle supported surface mesoporous silica nanocomposites (mSiO2@NH2@Ag) were characterized with FT-IR, X-ray photoelectron spectroscopy, X-ray diffraction, scanning electron microscopy and high-resolution transmission electron microscopy. Antibacterial activities of the synthesized mSiO2@NH2@Ag were investigated against Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (SAU) using the conventional plate-count method. The results demonstrated that the synthesized nanocomposites exhibited excellent antibacterial properties against E. coli and SAU. Furthermore, because of the slow release property of silver, the synthesized nanocomposites can be used as an economic recyclable material in various antibacterial applications, such as water purification systems and environmental control of bacteria.
1. Introduction
Bacterial infection is a global problem concerning public security and health.1–3 Many approaches have been discussed to deal with bacteria's antibiotic resistance and many antibacterial agents, especially inorganic nanoparticles,4 e.g. gold,5 titanium,6,7 copper,8 magnesium oxide,9 zinc oxide10,11 and silver,2,12–14 have been used to reduce the risk to people's health.15–17 Significant studies indicated that silver nanoparticles have strong antibacterial effects because of their high antibacterial activity and broad antibacterial spectrum even at low concentrations,18–20 and relative nontoxicity to human cells. Furthermore, silver nanoparticles can be easily embedded within inorganic substrates and these silver nanoparticle containing materials are capable of the slow release of silver over an extended period, which is superior to conventional organic antibacterial materials with regards to safety, stability and prolonged antibacterial activities,.21 At present, zeolite,22,23 clay,24 calcium phosphate,25 silica26 and mesoporous materials27–32 have been reported as supports for fabricating silver-containing antibacterial agents. However, the limitation of these silver-containing antibacterial materials is that most silver nanoparticles were doped in the inner substrates and the inner silver nanoparticles may not contribute to the antibacterial effect at all. Surface mesoporous silica material with mesopore channels perpendicular to the core surface is expected to be a good candidate for idea inorganic substrates owing to its unique perpendicular orientation mesoporous channels structures and large surface area.33–35 These open perpendicular mesoporous channels could conveniently dope high dense silver nanoparticles at the outer channels and improve the antibacterial efficiency. However, there was little report about the surface mesoporous silica material based silver nanoparticles for antibacterial applications.In this study, we have developed an effective route to prepare silver nanoparticles-supported surface mesoporous silica nanocomposites for antibacterial applications. First, we fabricated core–shell structured surface mesoporous microspheres with SiO2 particles cores and perpendicularly aligned mesoporous silica shells (mSiO2). Then, 3-aminopropyltriethoxysilane (APTS) was modified onto the mSiO2 to graft amino functional groups onto the surface mesopore channels (mSiO2@NH2). The amino groups of APTS on the surface of mesopore channels provided the active site for the absorption of Ag+ through complexation interaction and increased the loading amount of silver ions on the mSiO2. The silver nanoparticles were directly created on the surface of mSiO2 by in situ chemical reduction of Ag precursor using ultrasonic wave reaction method. The synthesized silver nanoparticle supported mesoporous silica (mSiO2@NH2@Ag) was examined against Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (SAU) for their antibacterial efficacy.
2. Experimental
2.1. Reagents
Tetraethoxysilane (TEOS), anhydrous ethanol, ammonium hydroxide (25 wt% NH3 in water), and stearyltrimethyl ammonium bromide (STAB) were obtained from Beijing Chemical Company. AgNO3 and 3-aminopropyltriethoxysilane (APTS) purchased from Sigma-Aldrich and used as supplied. All other reagents were commercially available as analytical reagent grade.2.2. Preparation of mSiO2@NH2 microspheres
The core–shell mSiO2 microspheres were synthesized according to our previous work.35,36 Briefly, 0.2 g SiO2 (∼130 nm) particles were dispersed in mixed solution containing of STAB (0.67 g, 1.78 mmol), concentrated ammonia aqueous solution (0.6 mL, 28 wt%), deionized water (60 mL) and ethanol (30 mL). The mixed solution was stirred for 0.5 h and then 1.1 mL distilled TEOS was added. After the reaction for 12 h at room temperature, the suspension was centrifuged and washed with ethanol. The obtained white precipitate was vacuum dried at 343 K overnight and then was further calcined at 823 K for 6 h in air in order to remove STAB from the composites. 0.1 g of as-synthesized mSiO2 microspheres and 1 mL of APTS were added into anhydrous toluene to make 50 mL of mixture solution. The mixture was refluxed for 18 h under dry nitrogen. The resulting mSiO2@NH2 microspheres were separated by centrifuge, washed with toluene, ethanol and water in turn, and then vacuum dried at 343 K overnight for use.2.3. Preparation of mSiO2@NH2@Ag
1.0 g of the as-prepared mSiO2@NH2 microspheres were suspended in a series of 60 mL AgNO3 aqueous solution with different concentrations (0.5, 1.0, 2.0, 2.5, 3.0 M) in brown bottles and the mixed solution were ultrasonic treated for 2 h under dark condition, then were stirred at 313 K for another 2 hours. The silver(I) ions were reduced to silver nanoparticles on the mesopore channels of mSiO2@NH2 surface. The precipitate was separated by filtration and washed with deionized water to remove AgNO3 on the external surface. Finally, the composites were dried in vacuum at 343 K for 12 h obtained gray-colored mSiO2@NH2@Ag nanocomposites. The mSiO2@Ag without APTS modification procedure was prepared by the same procedure above and used for control antibacterial activity experiment.2.4. Experimental measurement method
The transmission electron microscopy (TEM) images were obtained on the JEOL-2010 transmission electron microscope at an accelerating voltage of 200 kV. The scanning electron microscopy (SEM) images were taken by using a field-emission scanning electron microscopy (FESEM, JEOL JSM-6700F, 10 kV). X-ray powder diffraction (XRD) pattern of the products was recorded on a D/max 2550 X-ray Diffractometer (RigaKu, Japan) using Cu Kα radiation (λ = 1.54178 Å). The FT-IR spectra in KBr were recorded using a Perkin-Elmer spectrometer (model GX2000). XPS analysis was carried out using a Thermo ESCALAB 250 equipped with a monochromatic microspot X-ray beam originating from the Al anode (Kα, X-ray at 1486.6 eV) with a spot diameter of 500 μM. The data were recorded at room temperature and under a pressure below 10−6 Pa.2.5. Microbiological experiment
The antibacterial activities of mSiO2@NH2@Ag were tested on E. coli as a Gram negative strain and SAU as a Gram positive strain. Both Gram negative and Gram positive bacteria were used for inhibitory zone tests, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests to investigate the antibacterial properties of mSiO2@NH2@Ag. A nutrient broth was used as the growing medium. Bacteria were grown aerobically in nutrient broth at 37 °C for 24 h.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio, and the final concentrations of the mSiO2@NH2@Ag were in a range of 0.5–0.0039 mg mL−1. Then, 0.2 mL of 109 CFU per mL E. coli or 108 CFU per mL SAU were added to above LB liquid medium containing different concentrations of mSiO2@NH2@Ag and incubated at 37 °C for 24 h with continuous agitation (180 rpm). The lowest concentration of the mSiO2@NH2@Ag that inhibits the visible growth of colony formation was defined as the MIC. The MBC was determined by subculturing 0.2 mL LB liquid from broth dilution without growth of bacteria in MIC tests to LB solid medium plate. After incubation at 37 °C for 24 h, the lowest concentration of the mSiO2@NH2@Ag that does not support colony formation was defined as the MBC.
1 volume ratio, and the final concentrations of the mSiO2@NH2@Ag were in a range of 0.5–0.0039 mg mL−1. Then, 0.2 mL of 109 CFU per mL E. coli or 108 CFU per mL SAU were added to above LB liquid medium containing different concentrations of mSiO2@NH2@Ag and incubated at 37 °C for 24 h with continuous agitation (180 rpm). The lowest concentration of the mSiO2@NH2@Ag that inhibits the visible growth of colony formation was defined as the MIC. The MBC was determined by subculturing 0.2 mL LB liquid from broth dilution without growth of bacteria in MIC tests to LB solid medium plate. After incubation at 37 °C for 24 h, the lowest concentration of the mSiO2@NH2@Ag that does not support colony formation was defined as the MBC.3. Results and discussion
3.1. Formation and characterization of mSiO2@NH2@Ag
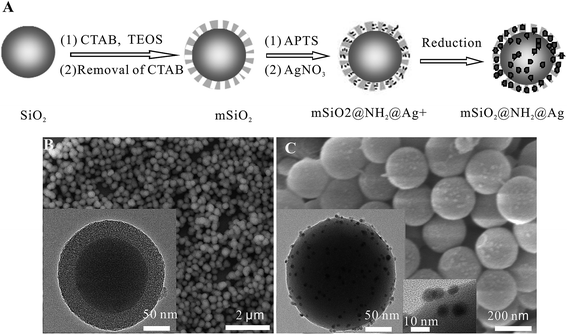
The preparation of the mSiO2@NH2@Ag was illustrated in Fig. 1A. The core–shell structured mSiO2 microspheres were produced by reacting TEOS with STAB surfactant at the surface of monodisperse SiO2 particles in the presence of basic ammonia aqueous condition according to our previous work.36 The aim of the modification with amino groups onto the mSiO2 microspheres was to absorb the maximum amount of Ag+ ions through the complex and electrostatic interactions between Ag+ ions and amino functional groups. Silver nanoparticles then were created by reducing Ag+ ions using ultrasonic wave reaction method. The created silver nanoparticles with high surface energy and unsaturated bonds were anchored on the surface and in the pores of mSiO2@NH2 by amino functional groups,29 denoted mSiO2@NH2@Ag. Fig. 1B and C showed SEM and TEM (insert) images of mSiO2. As seen in the TEM image, the ordered nanoporous SiO2 layers are uniformly coated on SiO2 core surface with thickness of ca. 30 nm and the nanopore channels are perpendicular to the microsphere surface. According to the result of the nitrogen adsorption–desorption isotherms analysis in our previous work,36 the BET surface area of the prepared mSiO2 here should be more than 400.0 m2 g−1. This perpendicular aligned nanoporous channels of mSiO2 not only offer high surface area for the load of a larger amount of silver nanoparticles, but also could improve the antibacterial efficiency, because these open perpendicular nanoporous channels make silver nanoparticles get in touch with bacteria conveniently. The SEM and TEM (insert) images of mSiO2@NH2@Ag in Fig. 1C showed that after the ultrasonic wave reaction process, Ag nanoparticles were loaded on the surface of the mSiO2@NH2 with the small sizes 4–10 nm. The ordered orientation channels in the outer layer of mSiO2@NH2@Ag were disappeared compared with the TEM image of mSiO2 in Fig. 1B, this may due to that the modification with amino groups and very small size Ag nanoparticles filled with the channels and the channels could not be distinguished.Fig. 2A showed the FT-IR transmission spectra for the prepared mSiO2, mSiO2@NH2 and mSiO2@NH2@Ag. From curve b of Fig. 2, we could see that the characteristic peaks of amino groups at the region of 1382–1565 cm−1 and the absorbance bands of stretching vibrations of –CH2 at the 2943 cm−1 could be observed after modification of APTS. However, these absorbance of the bands cannot observed on unmodified mSiO2 microspheres (Fig. 2A, curve a), which suggested that the amino functional groups has been banded with mesoporous silica surfaces.36 Compared with curve b, we could see those different O–H stretching vibrations in 3424 cm−1 and the peaks of amino groups at the region of 1382–1565 cm−1 weakened or disappeared in curve c after silver nanoparticles loaded onto mesoporous silica surface. This was attributed to the created silver nanoparticles transformated the terminal Si–OH groups into the Si–O–Ag network.29,37 The peak at 1385 cm−1 corresponding to NO3− ion in curve c indicated that there were a certain amount of silver ions existed in the mesopores surface.37
The mSiO2@NH2@Ag also were characterized by XPS spectrum and XRD analysis. As shown in Fig. 2B, the XPS spectrum of mSiO2@NH2@Ag together with that of the mSiO2@NH2 and mSiO2 were examined. For mSiO2@NH2, the XPS spectrum in the region of 392–410 eV of curve b showed an intense peak at 399.8 eV, which were the characteristic peaks of N 1s in NH2 groups.38 We can conclude that the amino functional groups have been modified onto the mesoporous silica surfaces because that the N can only be derived from the amino functional groups. For mSiO2@NH2@Ag, two bands at ca. 368.25 and 374.52 eV (Fig. 2B, insert) were observed. These two bands were ascribed to Ag 3d5/2 and Ag 3d3/2 binding energies of the metallic Ag,39,40 which confirmed the existence of metallic Ag in our mSiO2@NH2@Ag composite materials. Fig. 2C showed the room-temperature wide-angle XRD data of mSiO2@NH2@Ag (curve b) and mSiO2 (curve a). The broadened diffraction peak appeared at 20–32° in both curve a and b belonging to the amorphous porous SiO2 matrix. The diffraction peaks at 38.1°, 44.2°, 64.7° and 77.4° in curve b correspond to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) diffraction planes of cubic silver, respectively [JCPDS no. 4-0783],30 and these appear along with a broad peak in the 20–32° range for amorphous porous SiO2. These diffraction peaks indicated the presence of nanocrystalline Ag particles embedded on the porous SiO2 matrix. The influence of the AgNO3 concentrations to antibacterial activities in preparing mSiO2@NH2@Ag were investigated and the results showed that the antibacterial activities remained relatively constant when AgNO3 concentrations were above 2 M AgNO3. This may due to that Ag loading capacity was saturated when AgNO3 concentrations were more than 2 M, so we used 2 M AgNO3 to prepare mSiO2@NH2@Ag. The calculated silver content of the mSiO2@NH2@Ag was ca. 3.24 wt% from the ICP-OES measurement.
3.2. The antibacterial effect test
The antibacterial effect of prepared mSiO2@NH2@Ag was first evaluated by inhibitory zone tests against E. coli as a Gram negative strain and SAU as a Gram positive strain. The inhibition zones tests of mSiO2@NH2@Ag, mSiO2@Ag without APTS modification and mSiO2 against E. coli and SAU for 24 h were shown in Fig. 3. We could see that mSiO2@NH2@Ag showed large inhibition zones against both SAU and E. coli. The inhibition zone diameter (IZD) of mSiO2@NH2@Ag against SAU was 27 cm (Fig. 3A), and the IZD against E. coli was 17 cm (Fig. 3B). However, mSiO2@Ag without APTS modification procedure and mSiO2 had no inhibitory effect against SAU, and only had little inhibitory effect against E. coli. The results showed that mSiO2@NH2@Ag exhibited good antibacterial activity and inhibited the growth of SAU and E. coli effectively due to their large inhibition zones. The mSiO2@NH2@Ag showed excellent antibacterial properties, whereas the mSiO2 exhibited no bacterial inhibitory effects, suggesting that this antibacterial property was due to the presence of metal silver nanoparticles in the composite material. It has been reported that when the silver nanoparticles were dispersed throughout a silica matrix, Ag+ ions were released when the materials interact with an aqueous phase,13,41 and these Ag+ ions are responsible for the antibacterial activity of the materials.30,42–44 Compared the antibacterial activities of mSiO2@NH2@Ag and mSiO2@Ag without APTS modification procedure; we could see that APTS modification procedure play a very important role in the immobilization of silver nanoparticles onto mSiO2. Because the amino group of APTS could absorb a larger amount of Ag+ ions tightly through the complex and electrostatic interactions between Ag+ ions and amino functional groups, thus, a larger amount of Ag+ ions were absorbed onto mSiO2 and reduced to Ag nanoparticles through the next ultrasonic wave reaction method. However, there only a small amount of Ag+ ions could be absorbed onto mSiO2 without APTS modification and the created Ag nanoparticles also could easily break away from the channels of mSiO2 without amino functional groups' interactions during the filtration and washing procedure45 (TEM image of mSiO2@Ag find Ag nanoparticles hardly, not shown). So, the mSiO2@Ag without APTS modification procedure has little antibacterial activity. | ||
| Fig. 3 The inhibition zones test results of mSiO2, mSiO2@NH2 and mSiO2@NH2@AgNPs against (A) SAU (108 CFU) and (B) E. coli (109 CFU). | ||
To further study the antibacterial properties of mSiO2@NH2@Ag samples, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests against E. coli and SAU by tube dilution methods were also evaluated. A serial of doubling dilution of the mSiO2@NH2@Ag were prepared in the tubes and each dilution was inoculated with the Luria–Bertani (LB) liquid medium in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio, and the final concentration of the mSiO2@NH2@Ag in the tubes were in a range of 0.5–0.0039 mg mL−1. Then, 0.2 mL of 109 CFU per mL E. coli or 108 CFU per mL ASU were added to above LB liquid medium containing different concentrations of mSiO2@NH2@Ag and then incubated at 37 °C for 24 h with continuous agitation (180 rpm). The lowest concentration of mSiO2@NH2@Ag that inhibited the visible growth of colony formation was defined as the MIC. The MBC was determined by subculturing 0.2 mL LB liquid from broth dilution of above MIC tube to LB solid medium plate. After incubation at 37 °C for 24 h, the lowest concentration of the mSiO2@NH2@Ag that does not support colony formation was defined as the MBC. The experiments results showed that the MIC and MBC of mSiO2@NH2@Ag against SAU were 0.156 (the 6# tube in Fig. 4A, which equal to 5.06 μg mL−1 of Ag) and 0.3125 mg mL−1 (Fig. 5A, 10.12 μg mL−1 of Ag), respectively. In the case of E. coli, the MIC and MBC of mSiO2@NH2@Ag were found to be 0.3125 (the 5# tube in Fig. 4B, 10.12 μg mL−1 of Ag) and 0.625 mg mL−1 (Fig. 5C, 20.24 μg mL−1 of Ag). Significant studies showed that the major factor for the MIC and MBC of a material was the interaction of bacteria with active Ag,17,27,30 and several studies have reported in the literature involving silver-containing mesoporous silica compounds have shown that the MIC of silver particles for E. coli ranges from 2 to 75 μg mL−1 of Ag.30,46 For example, the MIC values for E. coli obtained for the Ag–SiO2 nanocomposite was 300 μg mL−1 (37.65 μg mL−1 of Ag);13 the MIC values for E. coli obtained for the Ag–SiO2 nanocomposite produced by Egger et al. was 62.5 μg mL−1 (12.5 μg mL−1 of Ag);46 the MIC values of AgCl–mesoporous silica (AgCl–SBA-15) and Ag–mesoporous silica nanocomposite (Ag–SBA-15) for E. coli were 25 μg Ag mL−1 and 100 μg (ref. 30) and 203 μg Ag mL−1 (ref. 47), respectively; the Ag–mesoporous silica nanocomposite (Ag–MCM-41) for E. coli were 258.5 μg Ag mL−1.47 Compared with above-mentioned silver-containing mesoporous silica compounds, the present mSiO2@NH2@Ag exhibited more excellent antibacterial activities according to the Ag contents in the materials. The excellent antibacterial activity of mSiO2@NH2@Ag was due to the mSiO2@NH2@Ag could offer high surface area for the load of a larger amount of silver nanoparticles at the outer channels to improve the antibacterial efficiency, because these open perpendicular nanoporous channels make silver nanoparticles get in touch with bacteria conveniently.
1 volume ratio, and the final concentration of the mSiO2@NH2@Ag in the tubes were in a range of 0.5–0.0039 mg mL−1. Then, 0.2 mL of 109 CFU per mL E. coli or 108 CFU per mL ASU were added to above LB liquid medium containing different concentrations of mSiO2@NH2@Ag and then incubated at 37 °C for 24 h with continuous agitation (180 rpm). The lowest concentration of mSiO2@NH2@Ag that inhibited the visible growth of colony formation was defined as the MIC. The MBC was determined by subculturing 0.2 mL LB liquid from broth dilution of above MIC tube to LB solid medium plate. After incubation at 37 °C for 24 h, the lowest concentration of the mSiO2@NH2@Ag that does not support colony formation was defined as the MBC. The experiments results showed that the MIC and MBC of mSiO2@NH2@Ag against SAU were 0.156 (the 6# tube in Fig. 4A, which equal to 5.06 μg mL−1 of Ag) and 0.3125 mg mL−1 (Fig. 5A, 10.12 μg mL−1 of Ag), respectively. In the case of E. coli, the MIC and MBC of mSiO2@NH2@Ag were found to be 0.3125 (the 5# tube in Fig. 4B, 10.12 μg mL−1 of Ag) and 0.625 mg mL−1 (Fig. 5C, 20.24 μg mL−1 of Ag). Significant studies showed that the major factor for the MIC and MBC of a material was the interaction of bacteria with active Ag,17,27,30 and several studies have reported in the literature involving silver-containing mesoporous silica compounds have shown that the MIC of silver particles for E. coli ranges from 2 to 75 μg mL−1 of Ag.30,46 For example, the MIC values for E. coli obtained for the Ag–SiO2 nanocomposite was 300 μg mL−1 (37.65 μg mL−1 of Ag);13 the MIC values for E. coli obtained for the Ag–SiO2 nanocomposite produced by Egger et al. was 62.5 μg mL−1 (12.5 μg mL−1 of Ag);46 the MIC values of AgCl–mesoporous silica (AgCl–SBA-15) and Ag–mesoporous silica nanocomposite (Ag–SBA-15) for E. coli were 25 μg Ag mL−1 and 100 μg (ref. 30) and 203 μg Ag mL−1 (ref. 47), respectively; the Ag–mesoporous silica nanocomposite (Ag–MCM-41) for E. coli were 258.5 μg Ag mL−1.47 Compared with above-mentioned silver-containing mesoporous silica compounds, the present mSiO2@NH2@Ag exhibited more excellent antibacterial activities according to the Ag contents in the materials. The excellent antibacterial activity of mSiO2@NH2@Ag was due to the mSiO2@NH2@Ag could offer high surface area for the load of a larger amount of silver nanoparticles at the outer channels to improve the antibacterial efficiency, because these open perpendicular nanoporous channels make silver nanoparticles get in touch with bacteria conveniently.
3.3. The reuse study
In order to evaluate the possibility of regeneration and reuse of the mSiO2@NH2@Ag nanocomposites, reuse experiments have been performed. The used mSiO2@NH2@Ag were washed and centrifuged five times with deionized water, and its antibacterial effect was evaluated by IZD test against E. coli and SAU. The results showed that the IZD against SAU and E. coli were still 20 cm and 14 cm, respectively (Fig. 6). For further evaluating the reuse possibility, the successive 5 reuse cycles experiments were performed and found the IZD test against E. coli and SAU were 15.7 and 11.6 cm in 5th cycle, respectively, the silver content of mSiO2@NH2@Ag after 5 cycles use and washing was 2.68% according the ICP-OES measurement. These showed that mSiO2@NH2@Ag had slow release property and could be used as a recyclable material. Moreover, the stabilization experiment of Ag concentration in 0.05 M Tris–HCl buffer solution (pH 7.4) at 37 °C against the immersion time was also studied. As shown in Fig. 7, the release rate of Ag was relatively high during the initial 16 hours and then stayed almost constant. The slow release property of Ag nanoparticles from the mSiO2 layers was due to the collective coordination effect of large surface area of mSiO2 and the amino functional groups' complex interactions, and this ensure the used mSiO2@NH2@Ag could be reused when it was applied in water pollution problem. | ||
| Fig. 7 Ag+ release from mSiO2@NH2@Ag into Tris–HCl solution (pH 7.4) at 37 °C against immersion time. | ||
4. Conclusions
In summary, we have synthesized silver nanoparticles-supported surface mesoporous silica nanocomposites for antibacterial applications. The modification of APTS could affect the silver content and antibacterial activity of nanocomposites. The silver nanoparticles were directly created on the surface of mesoporous silica by in situ chemical reduction of Ag precursor using ultrasonic wave reaction method. The synthesized nanocomposites were examined against both Gram-negative E. coli and Gram-positive SAU for their antibacterial efficacy. The results demonstrated that the synthesized nanocomposites exhibited more excellent antibacterial properties than those reported silver-containing traditional mesoporous silica compounds. Because of the slow release property of Ag nanoparticles, the synthesized nanocomposites could be used as a recyclable material in various antibacterial applications, such as water purification systems and environmental control of bacteria.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant Nos 21377099, 21201132), the Excellent Young Support Program of Anhui Province (Huang & Fu, 2014), and the Science & Research Program of Anhui Province (No. 1206c0805031, 1406c085021). We also thank Mr Meng Chen of West Anhui University for his contribution work of the stabilization experiment of sliver.References
- E. R. Kenawy, S. D. Worley and R. Broughton, Biomacromolecules, 2007, 8, 1359–1384 CrossRef CAS PubMed.
- A. Kumar, P. K. Vemula, P. M. Ajayan and G. John, Nat. Mater., 2008, 7, 236–241 CrossRef CAS PubMed.
- M. J. Saif, J. Anwar and M. A. Munawar, Langmuir, 2009, 25, 377–379 CrossRef CAS PubMed.
- M. A. Vargas-Reus, K. Memarzadeh, J. Huang, G. G. G. Ren and R. P. Allaker, Int. Arabic J. Antimicrob. Agents, 2012, 40, 135–139 CrossRef CAS PubMed.
- R. Bhattacharya and P. Mukherjee, Adv. Drug Delivery Rev., 2008, 60, 1289–1306 CrossRef CAS PubMed.
- M. V. Liga, E. L. Bryant, V. L. Colvin and Q. L. Li, Water Res., 2011, 45, 535–544 CrossRef CAS PubMed.
- S. G. Chen, Y. J. Guo, H. Q. Zhong, S. J. Chen, J. A. Li, Z. C. Ge and J. N. Tang, Chem.–Eng. J., 2014, 256, 238–246 CrossRef CAS PubMed.
- J. P. Ruparelia, A. K. Chatteriee, S. P. Duttagupta and S. Mukherji, Acta Biomater., 2008, 4, 707–716 CrossRef CAS PubMed.
- T. Jin and Y. P. He, J. Nanopart. Res., 2011, 13, 6877–6885 CrossRef CAS.
- J. Pasquet, Y. Chevalier, E. Couval, D. Bouvier, G. Noizet, C. Morliere and M. A. Bolzinger, Int. J. Pharm., 2014, 460, 92–100 CrossRef CAS PubMed.
- M. Eshed, J. Lellouche, A. Gedanken and E. Banin, Adv. Funct. Mater., 2014, 24, 1382–1390 CrossRef CAS PubMed.
- S. K. Rastogi, V. J. Rutledge, C. Gibson, D. A. Newcombe, J. R. Branen and A. L. Branen, J. Nanomed. Nanotechnol., 2011, 7, 305–314 CrossRef CAS PubMed.
- Y. H. Kim, D. K. Lee, H. G. Cha, C. W. Kim and Y. S. Kang, J. Phys. Chem. C, 2007, 111, 3629–3635 CAS.
- F. A. Sheikh, H. W. Ju, B. M. Moon, H. J. Park, J. H. Kim, O. J. Lee and C. H. Park, J. Biomed. Mater. Res., Part A, 2014, 102, 3459–3469 CrossRef PubMed.
- M. Saleem, M. Nazir, M. S. Ali, H. Hussain, Y. S. Lee, N. Riaz and A. Jabbar, Nat. Prod. Rep., 2010, 27, 238–254 RSC.
- W. G. I. U. Rathnayake, H. Ismail, A. Baharin, I. M. C. C. D. Bandara and S. Rajapakse, J. Appl. Polym. Sci., 2014, 131, 39601–39608 Search PubMed.
- C. Marambio-Jones and E. M. V. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS PubMed.
- P. Li, J. Li, C. Z. Wu, Q. S. Wu and J. Li, Nanotechnology, 2005, 16, 1912–1917 CrossRef CAS.
- M. Singh, S. Singh, S. Prasad and I. S. Gambhir, Digest Journal of Nanomaterials and Biostructures, 2008, 3, 115–122 Search PubMed.
- A. Top and S. Ulku, Appl. Clay Sci., 2004, 27, 13–19 CrossRef CAS PubMed.
- B. Kwakye-Awuah, I. Radecka, M. A. Kenward and C. D. Williams, J. Chem. Technol. Biotechnol., 2008, 83, 1255–1260 CrossRef CAS PubMed.
- B. Kwakye-Awuah, C. Williams, M. A. Kenward and I. Radecka, J. Appl. Microbiol., 2008, 104, 1516–1524 CrossRef CAS PubMed.
- E. Abdullayev, K. Sakakibara, K. Okamoto, W. B. Wei, K. Ariga and Y. Lvov, ACS Appl. Mater. Interfaces, 2011, 3, 4040–4046 CAS.
- J. S. Lee and W. L. Murphy, Adv. Mater., 2013, 25, 1173–1179 CrossRef CAS PubMed.
- H. S. Jia, W. S. Hou, L. Q. Wei, B. S. Xu and X. G. Liu, Dent. Mater., 2008, 24, 244–249 CrossRef CAS PubMed.
- H. Yang, Y. Liu, Q. H. Shen, L. F. Chen, W. H. You, X. M. Wang and J. S. Sheng, J. Mater. Chem., 2012, 22, 24132–24138 RSC.
- D. Carmona, P. Lalueza, F. Balas, M. Arruebo and J. Santamaria, Microporous Mesoporous Mater., 2012, 161, 84–90 CrossRef CAS PubMed.
- D. V. Quang, P. B. Sarawade, A. Hilonga, J. K. Kim, Y. G. Chai, S. H. Kim, J. Y. Ryu and H. T. Kim, Colloids Surf., A, 2011, 389, 118–126 CrossRef CAS PubMed.
- B. Naik, V. Desai, M. Kowshik, V. S. Prasad, G. F. Fernando and N. N. Ghosh, Particuology, 2011, 9, 243–247 CrossRef CAS PubMed.
- Y. I. Seo, Y. J. Lee, D. G. Kim, K. H. Lee and Y. Do Kim, Microporous Mesoporous Mater., 2011, 139, 211–215 CrossRef CAS PubMed.
- L. H. Dong, T. Liu, L. Zhang and Y. S. Yin, Appl. Chem. Eng., 2011, 236–238, 1775–1778 CAS.
- Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 2008, 130, 28–31 CrossRef CAS PubMed.
- S. B. Yoon, J. Y. Kim, J. H. Kim, Y. J. Park, K. R. Yoon, S. K. Park and J. S. Yu, J. Mater. Chem., 2007, 17, 1758–1761 RSC.
- X. C. Fu, J. Wu, C. G. Xie, Y. Zhong and J. H. Liu, Anal. Methods, 2013, 5, 2615–2622 RSC.
- X. C. Fu, X. Chen, J. Wang, J. H. Liu and X. J. Huang, Electrochim. Acta, 2010, 56, 102–107 CrossRef CAS PubMed.
- X. L. Li, W. W. Zuo, M. Luo, Z. S. Shi, Z. C. Cui and S. Zhu, Chem. Res. Chin. Univ., 2013, 29, 1214–1218 CrossRef CAS.
- H. L. Li, N. Perkas, Q. L. Li, Y. Gofer, Y. Koltypin and A. Gedanken, Langmuir, 2003, 19, 10409–10413 CrossRef CAS.
- M. S. Zhu, P. L. Chen and M. H. Liu, ACS Nano, 2011, 5, 4529–4536 CrossRef CAS PubMed.
- L. S. Zhang, K. H. Wong, Z. G. Chen, J. C. Yu, J. C. Zhao, C. Hu, C. Y. Chan and P. K. Wong, Appl. Catal., A, 2009, 363, 221–229 CrossRef CAS PubMed.
- T. Liu, X. Song, Z. W. Guo, Y. H. Dong, N. Guo and X. T. Chang, Colloids Surf., B, 2014, 116, 793–796 CrossRef CAS PubMed.
- S. H. Min, J. H. Yang, J. Y. Kim and Y. U. Kwon, Microporous Mesoporous Mater., 2010, 128, 19–25 CrossRef CAS PubMed.
- H. Yang, W. H. You, Q. H. Shen, X. M. Wang, J. S. Sheng, D. Cheng, X. D. Cao and C. C. Wu, RSC Adv., 2014, 4, 2793–2796 RSC.
- W. K. Jung, H. C. Koo, K. W. Kim, S. Shin, S. H. Kim and Y. H. Park, Appl. Environ. Microbiol., 2008, 74, 2171–2178 CrossRef CAS PubMed.
- Q. Shen, J. Wang, H. Yang, X. Ding, Z. Luo, H. Wang, C. Pan, J. Sheng and D. Cheng, J. Non-Cryst. Solids, 2014, 391, 112–116 CrossRef CAS PubMed.
- S. Egger, R. P. Lehmann, M. J. Height, M. J. Loessner and M. Schuppler, Appl. Environ. Microbiol., 2009, 75, 2973–2976 CrossRef CAS PubMed.
- A. Szegedi, M. Popova, K. Yoncheva, J. Makk, J. Mihaly and P. Shestakova, J. Mater. Chem. B, 2014, 2, 6283–6292 RSC.
| This journal is © The Royal Society of Chemistry 2015 |