Themed collection Electrocatalytic hydrogen evolution

Production of hydrogen by electrocatalysis: making the H–H bond by combining protons and hydrides
Electrocatalytic production of hydrogen by nickel complexes is reviewed, with an emphasis on heterocoupling of protons and hydrides.
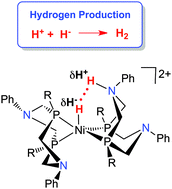
Chem. Commun., 2014,50, 3125-3143
https://doi.org/10.1039/C3CC46135A
Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts
An overview of transition-metal carbide and nitride materials as heterogeneous catalysts for electrochemical hydrogen evolution.
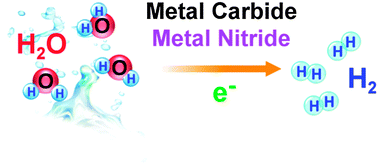
Chem. Commun., 2013,49, 8896-8909
https://doi.org/10.1039/C3CC44076A
Electrocatalytic hydrogen evolution from neutral water by molecular cobalt tripyridine–diamine complexes
A cobalt complex electrocatalyzes H2 production from neutral water, with an activity of 860 h−1 (cm2 Hg)−1 over 60 h at −1.25 V.
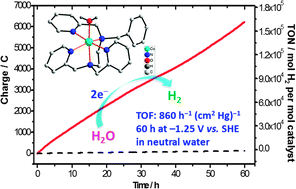
Chem. Commun., 2013,49, 9455-9457
https://doi.org/10.1039/C3CC43491E
Revealing and accelerating slow electron transport in amorphous molybdenum sulphide particles for hydrogen evolution reaction
A slow electron transport process in hydrogen evolution catalysed by amorphous molybdenum sulphides is revealed and then accelerated.
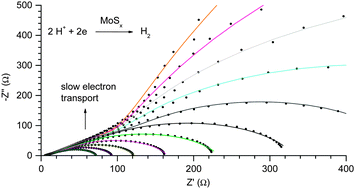
Chem. Commun., 2013,49, 8985-8987
https://doi.org/10.1039/C3CC45416A
Voltammetric and spectroscopic characterization of early intermediates in the Co(II)–polypyridyl-catalyzed reduction of water
Electrochemical detection of Co(III)-hydride and spectroscopic detection of Co(I) intermediates provide insight into the early processes within cobalt-catalyzed proton reduction.
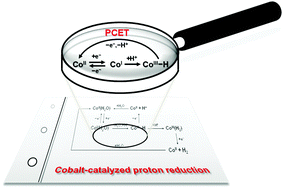
Chem. Commun., 2013,49, 8638-8640
https://doi.org/10.1039/C3CC44655G
Electric-field effects on the [FeFe]-hydrogenase active site
Electric field effects from the protein environment and an electrode are unravelled at the active site of [FeFe] hydrogenases by quantum calculations.
![Graphical abstract: Electric-field effects on the [FeFe]-hydrogenase active site](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3CC44112A&imageInfo.ImageIdentifier.Year=2013)
Chem. Commun., 2013,49, 8099-8101
https://doi.org/10.1039/C3CC44112A
Production of H2 at fast rates using a nickel electrocatalyst in water –acetonitrile solutions
The [Ni(PPh2NC6H4OH2)2]+2 electrocatalyst produces H2 solely from H3O+ in a mostly H2O environment at a rate of 170 000 s−1 and an overpotential of 470 mV.
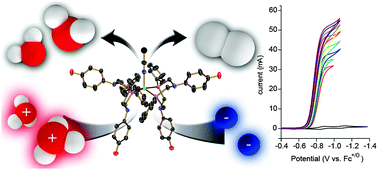
Chem. Commun., 2013,49, 7767-7769
https://doi.org/10.1039/C3CC43203C
Structural foundations for the O2 resistance of Desulfomicrobium baculatum [NiFeSe]-hydrogenase
Crystallographic analyses of an O2-resistant [NiFeSe]-hydrogenase show how O2 reacts with a selenocysteine and partially characterizes an enzyme activation pathway.
![Graphical abstract: Structural foundations for the O2 resistance of Desulfomicrobium baculatum [NiFeSe]-hydrogenase](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3CC43619E&imageInfo.ImageIdentifier.Year=2013)
Chem. Commun., 2013,49, 7061-7063
https://doi.org/10.1039/C3CC43619E
The mechanism of inhibition by H2 of H2-evolution by hydrogenases
We demonstrate that whether or not hydrogen evolution by FeFe or NiFe hydrogenase is significantly inhibited by H2 is not a consequence of active site chemistry, but rather relates to H2 transport within the enzyme.
Chem. Commun., 2013,49, 6840-6842
https://doi.org/10.1039/C3CC43297A
Catalytic hydrogen production by a Ni–Ru mimic of NiFe hydrogenases involves a proton-coupled electron transfer step
Hydrogen evolution from weak acids catalyzed by a structural mimic of the active site of NiFe hydrogenases [Ni(xbsms)Ru(C6Me6)Cl]+ proceeds through proton-coupled electron transfer steps.
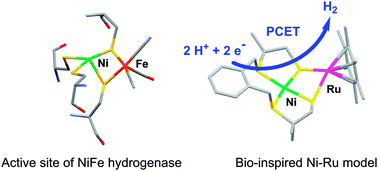
Chem. Commun., 2013,49, 5004-5006
https://doi.org/10.1039/C3CC40987B
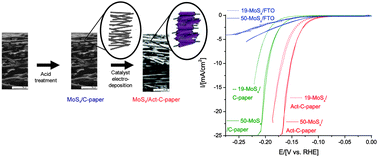
A high-porosity carbon molybdenum sulphide composite with enhanced electrochemical hydrogen evolution and stability
Long-term stability and hydrogen evolution activity of a highly active MoSx and acid modified porous and conductive carbon–fibre composite electrode.
Chem. Commun., 2013,49, 4965-4967
https://doi.org/10.1039/C3CC41945B
About this collection
This collection consists of a series of Communications from prominent scientists working on all aspects of electrocatalysis related to proton reduction, including, but not limited to: hydrogenases and bio-inspired mimics, new electrode materials, mechanistic studies (both at the experimental and theoretical level) and benchmarking.
This web theme issue will be a celebration of the current achievements and future perspectives in this exciting field of research. The guest editors of the issue are Vincent Artero (EA/CNRS/University of Grenoble), Fraser Armstrong (University of Oxford) and Jim Muckerman (Brookhaven National Laboratory).
Articles in this web themed issue will be added below as soon as possible after they are published. Please return to this page frequently to see the collection grow.