Electrocatalytic hydrogen evolution from neutral water by molecular cobalt tripyridine–diamine complexes†
Abstract
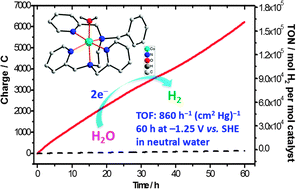
A cobalt complex with a tripyridine–diamine pentadentate ligand was found to be a highly active catalyst for electrochemical H2 production from neutral water, with an activity of 860 mol H2 (mol cat)−1 h−1 (cm2 Hg)−1 over 60 h CPE experiment at −1.25 V in a pH 7 phosphate buffer solution, without considerable deactivation.
- This article is part of the themed collection: Electrocatalytic hydrogen evolution

 Please wait while we load your content...
Please wait while we load your content...