Themed collection Most downloaded articles of 2017: Organic Chemistry

A unified photoredox-catalysis strategy for C(sp3)–H hydroxylation and amidation using hypervalent iodine
We report a unified photoredox-catalysis strategy for both hydroxylation and amidation of tertiary and benzylic C–H bonds.
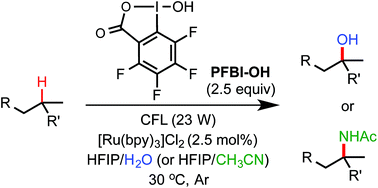
Chem. Sci., 2017,8, 7180-7185
https://doi.org/10.1039/C7SC02773G
Suzuki–Miyaura cross-coupling of amides and esters at room temperature: correlation with barriers to rotation around C–N and C–O bonds
We report the first general palladium-catalyzed Suzuki–Miyaura cross-coupling of both common amides and aryl esters through the selective cleavage of the C–N and C–O bonds at ambient temperature.
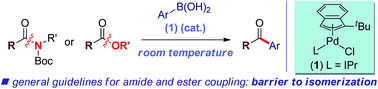
Chem. Sci., 2017,8, 6525-6530
https://doi.org/10.1039/C7SC02692G
Pyridine sulfinates as general nucleophilic coupling partners in palladium-catalyzed cross-coupling reactions with aryl halides
Pyridine sulfinates are stable and straightforward to prepare nucleophilic coupling partners for palladium catalyzed cross-coupling reactions with aryl and heteroaryl halides. The scope with respect to the halides coupling partner is considerable, and allows the preparation of a broad range of linked pyridines.
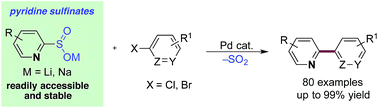
Chem. Sci., 2017,8, 4437-4442
https://doi.org/10.1039/C7SC00675F
Metal-free C–H alkylation of heteroarenes with alkyltrifluoroborates: a general protocol for 1°, 2° and 3° alkylation
A photoredox-catalyzed C–H functionalization of heteroarenes using a variety of primary, secondary, and tertiary alkyltrifluoroborates is reported.
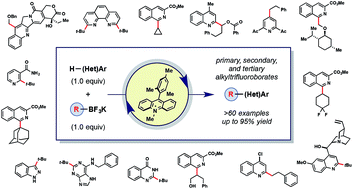
Chem. Sci., 2017,8, 3512-3522
https://doi.org/10.1039/C7SC00283A
Mild, visible light-mediated decarboxylation of aryl carboxylic acids to access aryl radicals
Herein we present the first example of aryl radical formation via the visible light-mediated decarboxylation of aryl carboxylic acids using photoredox catalysis.
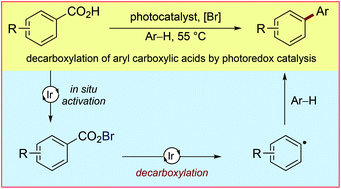
Chem. Sci., 2017,8, 3618-3622
https://doi.org/10.1039/C6SC05533H
Copper(I)-catalyzed sulfonylative Suzuki–Miyaura cross-coupling
A sulfonylative variant of a “classic” cross-coupling, with a broad substrate scope. The electrophilic trapping of a sulfinate intermediate was also possible.
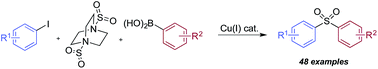
Chem. Sci., 2017,8, 3249-3253
https://doi.org/10.1039/C6SC05483H
A mild catalytic system for radical conjugate addition of nitrogen heterocycles
A catalytic redox system for the direct conjugate addition of pyridines and diazines to Michael acceptors has been developed.

Chem. Sci., 2017,8, 3121-3125
https://doi.org/10.1039/C7SC00243B
Room temperature decarboxylative cyanation of carboxylic acids using photoredox catalysis and cyanobenziodoxolones: a divergent mechanism compared to alkynylation
Conversion of carboxylic acids to nitriles using photoredox catalysis and benziodoxolone reagents: divergent mechanism when compared to alkynylation!
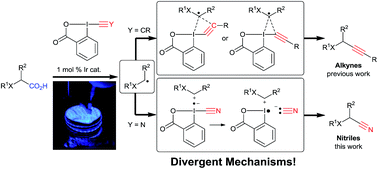
Chem. Sci., 2017,8, 1790-1800
https://doi.org/10.1039/C6SC04907A
One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides
A mild, efficient synthesis of sulfonyl fluorides from aryl and heteroaryl bromides utilizing palladium catalysis is described.
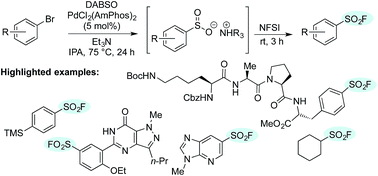
Chem. Sci., 2017,8, 1233-1237
https://doi.org/10.1039/C6SC03924C
Identification of monodentate oxazoline as a ligand for copper-promoted ortho-C–H hydroxylation and amination
The use of a weakly coordinating monodentate directing group for copper-mediated ortho-hydroxylation and amination reactions allows for the identification of an external oxazoline ligand as a promoter.
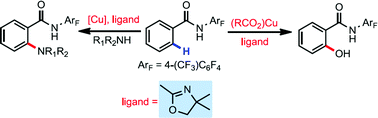
Chem. Sci., 2017,8, 1469-1473
https://doi.org/10.1039/C6SC03383K
About this collection
We are delighted to present a collection showcasing some of the most downloaded Chemical Science articles of 2017. This provides an easy way to quickly access the most important papers published in Chemical Science this year. These articles highlight exciting and impactful research across a broad range of subject areas, consisting of analytical chemistry, chemical biology, catalysis, energy, inorganic chemistry, materials science, nanoscience, organic chemistry and physical chemistry. We hope you enjoy reading this collection!