Mild, visible light-mediated decarboxylation of aryl carboxylic acids to access aryl radicals†
Abstract
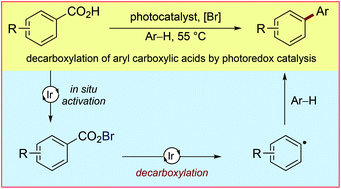
Herein we present the first example of aryl radical formation via the visible light-mediated decarboxylation of aryl carboxylic acids using photoredox catalysis. This method constitutes a mild protocol for the decarboxylation of cheap and abundant aryl carboxylic acids and tolerates both electron-rich substrates and those lacking ortho-substitution. The in situ formation of an acyl hypobromite is proposed to prevent unproductive hydrogen atom abstraction and trapping of the intermediate aroyloxy radical, enabling mild decarboxylation.
- This article is part of the themed collections: Celebrating our 2018 prize and award winners and Most downloaded articles of 2017: Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...