Themed collection p-Block Lewis Acids in Organic Synthesis

Introduction to p-block Lewis acids in organic synthesis
Guest editor Douglas Stephan introduces the Organic & Biomolecular Chemistry p-Block Lewis Acids in Organic Synthesis themed collection.

Org. Biomol. Chem., 2021,19, 7736-7736
https://doi.org/10.1039/D1OB90127C
Synthesis and reactivity of alkynyl boron compounds
Alkynyl boron compounds have attracted profound interest in synthetic organic chemistry. This review article summarizes the various methods developed for the synthesis and reactivity of alkynyl boron compounds in a chronological manner.

Org. Biomol. Chem., 2021,19, 7276-7297
https://doi.org/10.1039/D1OB00465D
Reactions promoted by hypervalent iodine reagents and boron Lewis acids
Understanding the role of boranes in hypervalent iodine chemistry will open up new reactivities which can be utilised in organic synthesis.
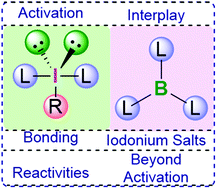
Org. Biomol. Chem., 2021,19, 4852-4865
https://doi.org/10.1039/D1OB00740H
Tris(pentafluorophenyl)borane catalyzed C–C and C–heteroatom bond formation
This review showcases a collective depiction on the potential utility of BCF as a versatile catalyst to develop various synthetic transformations.
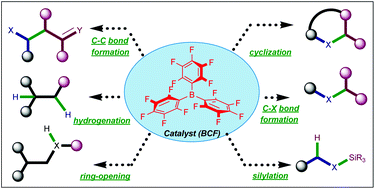
Org. Biomol. Chem., 2021,19, 1230-1267
https://doi.org/10.1039/D0OB02478C
Hydrosilylation and Mukaiyama aldol-type reaction of quinolines and hydrosilylation of imines catalyzed by a mesoionic carbene-stabilized borenium ion
Aldimines and ketimines are hydrosilylated with borenium catalysts at room temperature, giving the corresponding amines in excellent yields. For quinolines, subsequent Mukaiyama aldol reactions can be performed, which are also borenium-ion catalyzed.

Org. Biomol. Chem., 2021,19, 6786-6791
https://doi.org/10.1039/D1OB01056E
B(C6F5)3-catalyzed tandem protonation/deuteration and reduction of in situ-formed enamines
B(C6F5)3 exhibits high efficiency in tandem protonation/deuteration and reduction of in situ-formed enamines.

Org. Biomol. Chem., 2021,19, 4032-4036
https://doi.org/10.1039/D1OB00316J
FLP-catalysis meets hydrogen-bond activation
The potential of two chiral amidines and three non-chiral boranes in the metal-free hydrogen activation was explored.

Org. Biomol. Chem., 2020,18, 7321-7325
https://doi.org/10.1039/D0OB01492C
Ligand-free copper-catalyzed borylative defluorination: access to gem-difluoroallyl boronic acid derivatives
We report a ligand-free copper-catalyzed β-borylation, defluorination of β-substituted, α-trifluoromethyl-α,β-unsaturated esters.
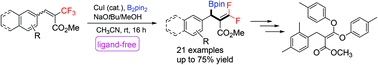
Org. Biomol. Chem., 2022,20, 366-374
https://doi.org/10.1039/D1OB01533H
An air-stable, Zn2+-based catalyst for hydrosilylation of alkenes and alkynes
An air-stable, Zn2+-based catalyst for catalytic hydrosilylation reactions of alkenes and alkynes.
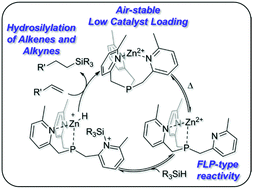
Org. Biomol. Chem., 2021,19, 5544-5550
https://doi.org/10.1039/D1OB00782C
Formation of amidino-borate derivatives by a multi-component reaction
Amidino-borate derivatives are formed in a five-component reaction between an olefin, three arylisocyanide equivalents and the dimethyl sulfide adduct of the bulky borane (Fmes)BH2.
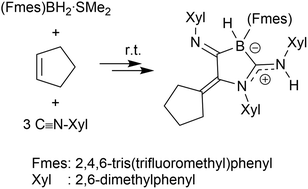
Org. Biomol. Chem., 2021,19, 5551-5554
https://doi.org/10.1039/D1OB00775K
Dibismuthanes in catalysis: from synthesis and characterization to redox behavior towards oxidative cleavage of 1,2-diols
A family of novel dimetallic Bi(III) and Bi(V) complexes is reported. Structural analysis of the complexes in the solid state permitted the study of the Bi–Bi distance and its impact in the catalytic redox properties.
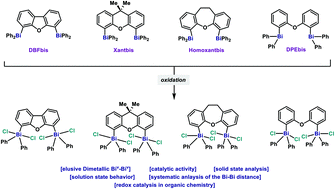
Org. Biomol. Chem., 2021,19, 4922-4929
https://doi.org/10.1039/D1OB00367D
Distiboranes based on ortho-phenylene backbones as bidentate Lewis acids for fluoride anion chelation
We report a new approach to fluorinated distiboranes by reaction of ortho-phenylene distibines with octafluorophenanthra-9,10-quinone. These new derivatives act as bidentate Lewis acids and readily chelate the fluoride anion.
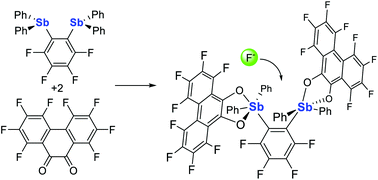
Org. Biomol. Chem., 2021,19, 4949-4957
https://doi.org/10.1039/D1OB00536G
The synthesis, properties, and reactivity of Lewis acidic aminoboranes
A series of aminoboranes were studied to understand their Lewis acidic properties. Unexpectedly, the relative Lewis acidity was dependent on the aromatic nature of the amino substituent and not the B–N bond π-overlap.
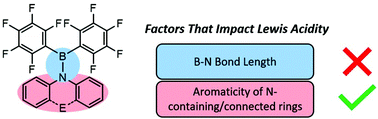
Org. Biomol. Chem., 2021,19, 4796-4802
https://doi.org/10.1039/D1OB00520K
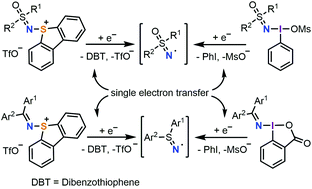
5-(Diarylimino)- and 5-(sulfoximido)dibenzothiophenium triflates: syntheses and applications as electrophilic aminating reagents
A new method has been developed for the synthesis of dibenzothiophenium salts containing diarylimino and sulfoximido substituents. The reactivity of these compounds under photochemical conditions is presented.
Org. Biomol. Chem., 2021,19, 2941-2948
https://doi.org/10.1039/D1OB00285F
Controlling selectivity in N-heterocycle directed borylation of indoles
N-Heterocycle directing groups lead to selective borylation of indole at C2 or C7 controlled by heterocycle ring size. With five membered heterocycle directing groups, C2 borylation is disfavoured due to an increased degree of distortion.

Org. Biomol. Chem., 2021,19, 2949-2958
https://doi.org/10.1039/D1OB00018G
Lewis acid-mediated synthesis of mono- and tris-indole adducts from chiral aziridines
Lewis acid-mediated regio- and stereoselective nucleophilic addition of 2- (or) 3-substituted indoles to non-activated aziridine 2-carboxaldehydes afforded mono- and tris-indole adducts.

Org. Biomol. Chem., 2020,18, 9473-9482
https://doi.org/10.1039/D0OB01865A
About this collection
This collection, guest edited by Professor Douglas Stephan (University of Toronto) aims to celebrate p-Block Lewis Acids in Organic Synthesis. The scope covers chemistry that exploits p-block elements for synthetic advantage, including catalytic or stoichiometric reactions that are of synthetic utility or potential.
New articles will be added to the collection upon publication. Please return to this page frequently to see the collection grow