An air-stable, Zn2+-based catalyst for hydrosilylation of alkenes and alkynes†
Abstract
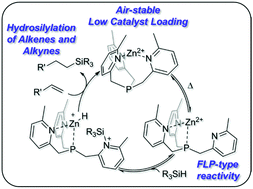
Hydrosilylation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double and C
C double and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bonds is one of the most widely used processes in organosilicon chemistry, mostly catalyzed by Pt-based complexes. We report here the synthesis of an air-stable dicationic Zn2+-based complex in a hemilabile tris(2-methyl-6-pyridylmethyl) phosphine (TmPPh) ligand, 12+[B(C6F5)4]2. When heated, 12+[B(C6F5)4]2 activates Si–H bonds reversibly via ligand/metal cooperation between Lewis acidic Zn2+ and Lewis basic N centers in a frustrated Lewis pair (FLP) type fashion. Consequently, 12+[B(C6F5)4]2 was found to be an effective catalyst for hydrosilylation reactions of C
C triple bonds is one of the most widely used processes in organosilicon chemistry, mostly catalyzed by Pt-based complexes. We report here the synthesis of an air-stable dicationic Zn2+-based complex in a hemilabile tris(2-methyl-6-pyridylmethyl) phosphine (TmPPh) ligand, 12+[B(C6F5)4]2. When heated, 12+[B(C6F5)4]2 activates Si–H bonds reversibly via ligand/metal cooperation between Lewis acidic Zn2+ and Lewis basic N centers in a frustrated Lewis pair (FLP) type fashion. Consequently, 12+[B(C6F5)4]2 was found to be an effective catalyst for hydrosilylation reactions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double and C
C double and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bonds. Remarkably, these hydrosilylation reactions can be loaded under aerobic conditions, as well as, in some cases, work under neat conditions. The mechanism of the activation of the Si–H bond and the hydrosilylation reaction is proposed based on experiments and density functional theory (DFT) calculations.
C triple bonds. Remarkably, these hydrosilylation reactions can be loaded under aerobic conditions, as well as, in some cases, work under neat conditions. The mechanism of the activation of the Si–H bond and the hydrosilylation reaction is proposed based on experiments and density functional theory (DFT) calculations.
- This article is part of the themed collections: Editor’s Collection, p-Block Lewis Acids in Organic Synthesis and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...