Themed collection Foldamer Chemistry

Intramolecular chiral communication in peptide –dendron hybrids
Intramolecular chirality transfer, amplification and solvent-mediated switching was observed in a series of random-coil peptide–dendron hybrids.
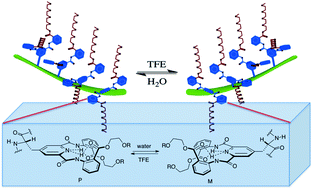
Org. Biomol. Chem., 2012,10, 2377-2379
https://doi.org/10.1039/C2OB07014F
Self-association-driven transition of the β-peptidic H12 helix to the H18 helix
Foldamer sequences constructed by using trans-ABHC and β3-hSer residues produce β-H18 helix in a solvent- and concentration-dependent way.
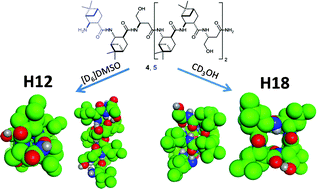
Org. Biomol. Chem., 2012,10, 255-259
https://doi.org/10.1039/C1OB06627G
Z-Formamidoximes in molecular folding and macrocycles
The formamidoxime Z isomer coupled with the pyridylbiscarboxamide conformational codon fold into planar, curved units prone to self-assembly into channel-like structures, as examplified with original macrocycles.
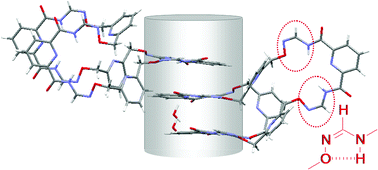
Org. Biomol. Chem., 2011,9, 7647-7651
https://doi.org/10.1039/C1OB06378B
ortho-Phenylene oligomers with terminal push–pull substitution
Substituent effects on conformational behavior and electronic spectra have been examined in the first series of push–pull o-phenylenes.
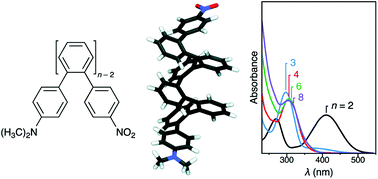
Org. Biomol. Chem., 2012,10, 3398-3405
https://doi.org/10.1039/C2OB07146K
Novel peptide foldameric motifs: a step forward in our understanding of the fully-extended conformation/310-helix coexistence
Fluorescent PyrAc-(Deg)n-O-(pNO2)Bzl peptides exist as mixtures of fully-extended (right) and 310-helical conformers, the latter typical of (Aib)n oligomers.
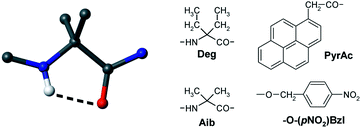
Org. Biomol. Chem., 2012,10, 2413-2421
https://doi.org/10.1039/C2OB06863J
Expedient synthesis of pseudo-Pro-containing peptides : towards constrained peptidomimetics and foldamers
The reaction of sulfonyl peptides containing L- or D-configured Ser or Thr with bis(succinimidyl) carbonate in the presence of a catalytic amount of a base affords, in solution or in the solid phase, the corresponding peptides with one or two, consecutive or alternate oxazolidin-2-ones (Oxd).
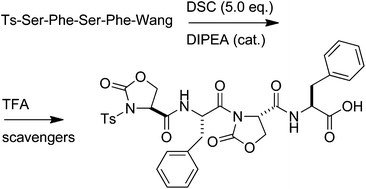
Org. Biomol. Chem., 2012,10, 2307-2317
https://doi.org/10.1039/C2OB07172J
Solid state NMR studies of oligourea foldamers: Interaction of 15N-labelled amphiphilic helices with oriented lipid membranes
The 15N-chemical shift tensor of urea bonds is determined and the bilayer topology of an antimicrobial oligourea analysed.
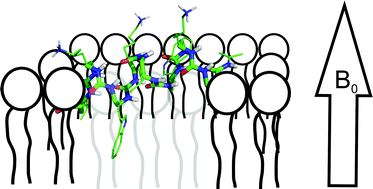
Org. Biomol. Chem., 2012,10, 1440-1447
https://doi.org/10.1039/C1OB06278F
Conformational stability of collagen triple helices functionalized in the Yaa position by click chemistry
Click chemistry was used to introduce moieties as sterically demanding as monosaccharides into the Yaa position of collagen model peptides. The effect of different triazolyl derivatives as well as the configuration of the functionalized proline residue on the thermal stability of the collagen triple helices was examined.
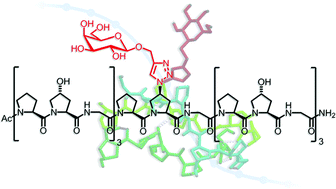
Org. Biomol. Chem., 2012,10, 1982-1986
https://doi.org/10.1039/C2OB06720J
Trichogin GA IV: A versatile template for the synthesis of novel peptaibiotics
One or more Gly-to-Lys replacements on the hydrophilic face of the peptide-template trichogin GA IV modulate its biological properties and promote a pH-mediated, reversible, 310- to α-helix transition.
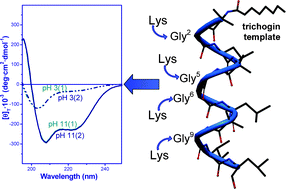
Org. Biomol. Chem., 2012,10, 1285-1299
https://doi.org/10.1039/C1OB06178J
Synthesis, structural investigation and computational modelling of water-binding aquafoldamers
A series of water-binding aquafoldamers have been studied in detail, illustrating their potential use for recognizing larger water clusters of diverse topologies and as synthetic water channels.
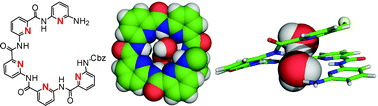
Org. Biomol. Chem., 2012,10, 1172-1180
https://doi.org/10.1039/C1OB06609A
Conformational stability studies of a stapled hexa-β3 -peptide library
An extensive library of new, 14-helical, hexa β3-peptides demonstrates that appropriately stapled hexa-β3-peptides can allow for a number of variations, including staple size, location and functionalization, without significant perturbation of the 14-helix.
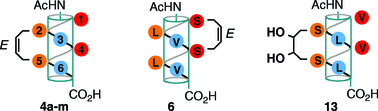
Org. Biomol. Chem., 2012,10, 1802-1806
https://doi.org/10.1039/C2OB06617C
Designing hybrid foldamers: the effect on the peptide conformational bias of β- versus α- and γ-linear residues in alternation with (1R,2S)-2-aminocyclobutane-1-carboxylic acid
Conformational bias can be tuned from ribbon-type structures to β-sheet-like and helical motifs by intercalating suitable linear segments between cis-cyclobutane residues
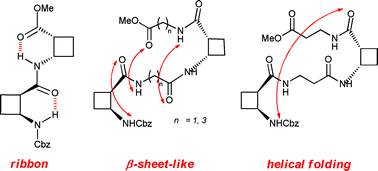
Org. Biomol. Chem., 2012,10, 861-868
https://doi.org/10.1039/C1OB06575K
Effect of capping groups at the N- and C-termini on the conformational preference of α,β-peptoids
Different capping groups have significant effects on spectral features/conformation of α,β-peptoids by CD, IR and NMR. These are most pronounced for shorter oligomers and are solvent dependent. This underlines the importance of considering capping groups for conformational studies.
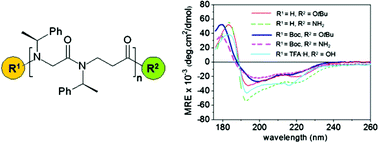
Org. Biomol. Chem., 2012,10, 1108-1122
https://doi.org/10.1039/C1OB06386C
β-Phenylproline: the high β-turn forming propensity of proline combined with an aromatic side chain
The β-phenyl group attached to proline does not disrupt the β-turn propensity of the natural amino acid.

Org. Biomol. Chem., 2012,10, 640-651
https://doi.org/10.1039/C1OB06561K
Flexible oligocholate foldamers as membrane transporters and their guest-dependent transport mechanism
Flexible oligocholate foldamers transport carboxyfluorescein and glucose across lipid membranes in distinctively different mechanisms.

Org. Biomol. Chem., 2012,10, 260-266
https://doi.org/10.1039/C1OB06364B
Design and synthesis of trans-3-aminopyran-2-carboxylic acid (APyC) and α/β-peptides with 9/11-helix
The participation of pyran oxygen of trans-3-aminopyran-2-carboxylic acid (APyC) in α/β-peptides resulting in 9/11-helix is envisaged to provide scope for new designs.
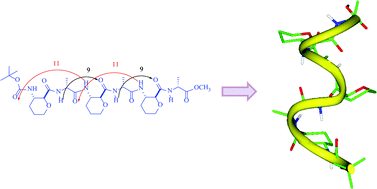
Org. Biomol. Chem., 2011,9, 8102-8111
https://doi.org/10.1039/C1OB06279D
meta-Substituted benzamide oligomers that complex mono-, di- and tricarboxylates: folding-induced selectivity and chirality
Benzamide-derived oligomers fold into compact conformations to complex aromatic and aliphatic carboxylate anions though multiple intermolecular N–H⋯O and C–H⋯O hydrogen bonds, and display helical chirality upon binding chiral glutamic acid dianion.
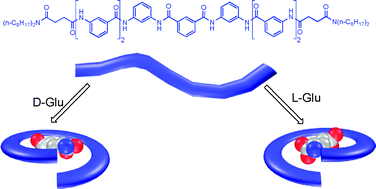
Org. Biomol. Chem., 2011,9, 8122-8129
https://doi.org/10.1039/C1OB06026K
Stereoselective preparation of β,γ-methano-GABA derivatives
The Kulinkovich–de Meijere reaction provides non-racemic trans cyclopropylamines, which are converted into enantiomerically pure N-Boc protected β,γ-methano-GABA derivatives.

Org. Biomol. Chem., 2011,9, 7517-7524
https://doi.org/10.1039/C1OB06095C
Synthesis of novel β-aminocyclobutanecarboxylic acid derivatives by a solvent -free aza–Michael addition and subsequent ring closure
A solvent-free aza–Michael addition leading to new substituted β-aminocyclobutanecarboxylic acid derivatives and the reactivity of these donor–acceptor substituted cyclobutanes is described.
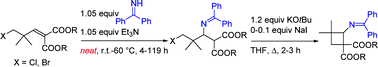
Org. Biomol. Chem., 2011,9, 7085-7091
https://doi.org/10.1039/C1OB05872J
The interaction of lipid modified pseudopeptides with lipid membranes
The conformational preferences of two lipopeptides Cn2H(2n+1)CO-L-Phe-D-Oxd-OBn or Cn2H(2n+1)CO-D-Phe-L-Oxd-OBn with n = 5 or 11 have been analyzed with solid-state 2H and 13C NMR techniques.
Org. Biomol. Chem., 2011,9, 6998-7006
https://doi.org/10.1039/C1OB05652B
Regio- and diastereoselective fluorination of alicyclic β-amino acids
Regio- and stereoselective approaches to fluorinated alicyclic β-amino esters has been developed.
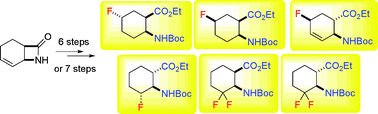
Org. Biomol. Chem., 2011,9, 6528-6534
https://doi.org/10.1039/C1OB05648D
About this collection
The peer-reviewed articles in this Organic & Biomolecular Chemistry's web theme issue highlight recent cutting-edge achievements in the exciting frontier field of Foldamer Chemistry.
The guest editor of this issue is Professor Ferenc Fulop (University of Szeged, Hungary). We hope that you will find this collection of articles enjoyable and interesting to read. Why not also view Professor Fulop's introduction to the web theme issue, available on our blog?