meta-Substituted arylamide trimer, pentamer and heptamer have been prepared from simple benzene-1,3-diamine, benzene-1,3-dicarboxylic acid, and 3-aminobenzoic acid units. 2D NOESY 1H NMR experiments reveal that these flexible oligomers form folded conformations to complex di- and tricarboxylate anions of varying sizes and shapes in DMSO of high polarity, which is driven by multiple intermolecular N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H⋯O
C and C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen-bonds between the amide and aromatic hydrogens of the oligomers and the carboxylate oxygens of the anions. Generally, tricarboxylate anions display an increased binding affinity compared with the dicarboxylate anions and the complexes formed by 1,3-benzenedicarboxylate anion are more stable than those formed by 1,2- or 1,4-benzenedicarboxylate anions. Circular dichroism experiments show that chiral glutamic acid dianion can induce the oligomers to produce chiral bias, leading to the formation of chiral supramolecular complexes.
C hydrogen-bonds between the amide and aromatic hydrogens of the oligomers and the carboxylate oxygens of the anions. Generally, tricarboxylate anions display an increased binding affinity compared with the dicarboxylate anions and the complexes formed by 1,3-benzenedicarboxylate anion are more stable than those formed by 1,2- or 1,4-benzenedicarboxylate anions. Circular dichroism experiments show that chiral glutamic acid dianion can induce the oligomers to produce chiral bias, leading to the formation of chiral supramolecular complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H⋯O
C and C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen-bonds between the
C hydrogen-bonds between the 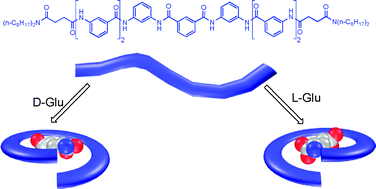

 Please wait while we load your content...
Please wait while we load your content...