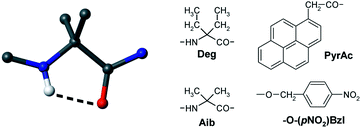
The fully-extended, multiple C5, conformation or 2.05 helix is a very appealing peptide secondary structure, in particular for its potential use as a molecular spacer, as it is characterized by the longest elevation (as high as 3.62 Å) between the α-carbon atoms of two consecutive α-amino acids. Despite this intriguing property, however, it is only poorly investigated and understood. Here, using a complete series of Cα,α-diethylglycine (Deg) homo-oligopeptide esters to the pentamer level, we exploited the properties of a fluorophore and a quencher, synthetically positioned at the N- and C-termini of the main chain, respectively, to check the applicability of the fully-extended conformation as a rigid molecular spacer. The fluorescence study was complemented by FT-IR absorption and NMR conformational investigations. The X-ray diffraction structures of selected compounds are also reported. Unfortunately, we find that, even in a solvent of low polarity, such as chloroform, in this peptide series an equilibrium does take place between the fragile fully-extended conformation and the 310-helical structure, the latter becoming more and more stable as the main chain is elongated. Since the Deg homo-peptide esters lacking any terminal aromatic group, previously investigated, are known to adopt a stable fully-extended conformation in chloroform solution, we tend to attribute the 3D-structure instability observed in this work to the presence of multiple aromatic rings in their blocking groups.

 Please wait while we load your content...
Please wait while we load your content...