Themed collection Synthesis III

Finding function and form
Nat. Prod. Rep., 2014,31, 411-413
https://doi.org/10.1039/C4NP90008A
The ever-expanding role of asymmetric covalent organocatalysis in scalable, natural product synthesis
This Highlight provides a brief overview of covalent, asymmetric modes of organocatalysis and applications of scalable versions of these methods applied to the total synthesis of natural products.
Nat. Prod. Rep., 2014,31, 1318-1327
https://doi.org/10.1039/C4NP00025K
Efficient synthesis strategies by application of transition metal-catalyzed carbene/nitrene insertions into C–H bonds
This Highlight article provides an overview of recent total syntheses that are characterized by high efficiency and enabled through a neat application of transition metal-catalyzed insertions of carbenes and nitrenes into C–H bonds.
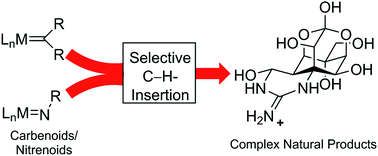
Nat. Prod. Rep., 2014,31, 449-455
https://doi.org/10.1039/C3NP70084D
Toward the ideal synthesis and molecular function through synthesis-informed design
This Highlight describes factors that contribute to an ideal synthesis, including economies (step, time, atom, solvent, energy) and orientations (target, diversity, safety, function), and the role of synthesis-informed design directed at function in advancing synthesis and its impact on science.
Nat. Prod. Rep., 2014,31, 433-440
https://doi.org/10.1039/C4NP00013G
sp3-sp3 Coupling reactions in the synthesis of natural products and biologically active molecules
This Highlight covers the current status of relatively unexplored sp3–sp3 cross-coupling reactions with particular focus on natural product and related syntheses.
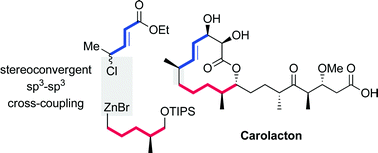
Nat. Prod. Rep., 2014,31, 441-448
https://doi.org/10.1039/C3NP70108E
Natural product synthesis in the age of scalability
In this Highlight, we discuss recent examples of natural product synthesis from our laboratory and others, where the preparation of gram-scale quantities of a target compound or a key intermediate allowed for a deeper understanding of biological activities or enabled further investigational collaborations. (Picture courtesy of Frank Fang of Eisai Inc.)
Nat. Prod. Rep., 2014,31, 419-432
https://doi.org/10.1039/C3NP70090A
Strategies towards the synthesis of calyciphylline A-type Daphniphyllum alkaloids
The structurally unique calyciphylline A-type Daphniphyllum alkaloids have recently attracted the interest of natural product synthesis groups, due to the challenge posed by the complex structural framework. Recently, several noteworthy core syntheses were reported using a variety of novel synthetic strategies, which include intramolecular Michael addition, Pd-catalysis, cycloaddition and Mannich-type reactions.
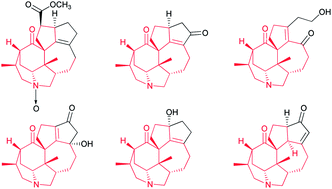
Nat. Prod. Rep., 2014,31, 550-562
https://doi.org/10.1039/C3NP70115H
The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis
This review will provide an overview on the recent developments of polyketide synthesis using the vinylogous Mukaiyama aldol reaction for the construction of advanced intermediates. In general, four different motifs can be constructed efficiently using the recent developments of asymmetric variants of this strategy.
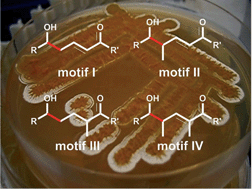
Nat. Prod. Rep., 2014,31, 563-594
https://doi.org/10.1039/C3NP70102F
Trying to rationalize total synthesis
The crux in multistep natural product synthesis is the exponential decrease of the yield with the number of steps. In this article various strategies are explained on how to circumvent this problem and to achieve efficient approaches nevertheless.
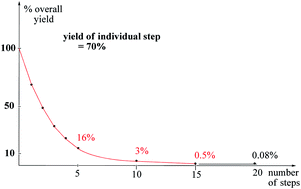
Nat. Prod. Rep., 2014,31, 595-603
https://doi.org/10.1039/C3NP70105K
Cu-mediated enamide formation in the total synthesis of complex peptide natural products
This review covers the synthetic assembly of peptide natural products in which a Cu-mediated C(sp2)–N bond formation is the key transformation, and particularly focuses on the total syntheses of cyclopeptide alkaloids, pacidamycin D, and yaku'amide A.
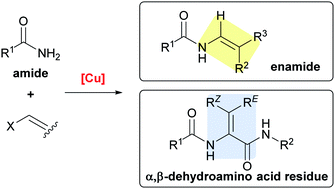
Nat. Prod. Rep., 2014,31, 514-532
https://doi.org/10.1039/C3NP70103D
Isolation, structural determination and synthetic approaches toward amphidinol 3
This review highlights the isolation and the structural determination of amphidinol 3 (AM3) with an emphasis on the different synthetic approaches.
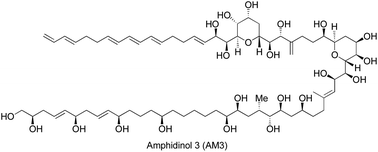
Nat. Prod. Rep., 2014,31, 468-488
https://doi.org/10.1039/C3NP70062C
Polyketide construction via hydrohydroxyalkylation and related alcohol C–H functionalizations: reinventing the chemistry of carbonyl addition
Direct alcohol C–H bond functionalization bypasses the use of chiral auxiliaries, premetallated C-nucleophiles, and discrete alcohol-to-aldehyde redox reactions. This technology has enabled syntheses of 6-deoxyerythronolide B, bryostatin 7, trienomycins A and F, cyanolide A, roxaticin.
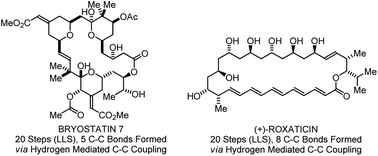
Nat. Prod. Rep., 2014,31, 504-513
https://doi.org/10.1039/C3NP70076C
Transition metal-promoted biomimetic steps in total syntheses
Important biomimetic steps in natural product synthesis have been promoted by transition metals, as exemplified by this beautiful ruthenium-catalyzed rearrangement of an endoperoxide into elysiapyrone A. Such reactions are supposed to occur during the biosynthesis, yet under different catalysis conditions.
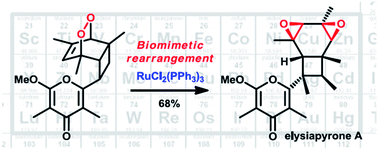
Nat. Prod. Rep., 2014,31, 533-549
https://doi.org/10.1039/C3NP70077A
Strategic innovation in the total synthesis of complex natural products using gold catalysis
This review has been organized from the perspective of synthetic target families, with emphasis on the use of gold-catalyzed transformations and cascade reactions that significantly increase molecular complexity.
Nat. Prod. Rep., 2014,31, 489-503
https://doi.org/10.1039/C3NP70075E
Sequential catalysis for stereoselective synthesis of complex polyketides
This review presents selected recent advances in sequential catalytic methods developed in our group for the rapid and stereoselective synthesis of key structural features of complex polyketides and their successful application in complex target synthesis.
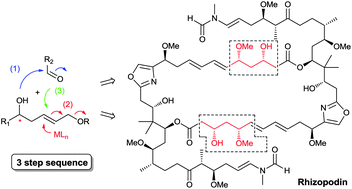
Nat. Prod. Rep., 2014,31, 456-467
https://doi.org/10.1039/C3NP70093C
Hot off the press
A personal selection of 32 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products such as thelepamide from Thelepus crispus.
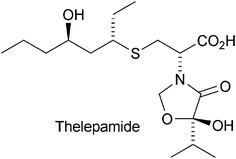
Nat. Prod. Rep., 2014,31, 414-418
https://doi.org/10.1039/C4NP90007C
About this collection
Natural Product Reports is delighted to present its latest themed collection dedicated to Synthesis. Guest-edited by Professor Dirk Trauner (Ludwig-Maximilians-University Munich), the collection features contributions from leaders in the field, with a specific focus on the role of transition metals in natural products synthesis.
Articles in this web themed issue will be added below as soon as possible after they are published. Please return to this page frequently to see the collection grow.
We hope you enjoy this selection!
Also of interest:
Synthesis, 2010
Synthesis, 2008