Cu-mediated enamide formation in the total synthesis of complex peptide natural products
Abstract
Covering: up to the end of 2013
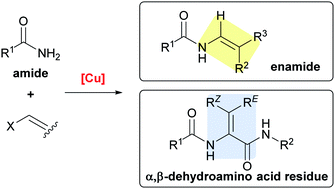
Cu-mediated C(sp2)–N bond formation has received intense interest recently, and has been applied to the total synthesis of a wide variety of structurally complex natural products. This review covers the synthetic assembly of peptide natural products in which Cu-mediated enamide formation is the key transformation. The total syntheses of cyclopeptide alkaloids, pacidamycin D, and yaku'amide A exemplify the versatility of the Cu-catalyzed cross-coupling reaction in comparison to other synthetic methods.
- This article is part of the themed collection: Synthesis III

 Please wait while we load your content...
Please wait while we load your content...