Themed collection Bunsentagung 2015: Solvation Science

Editorial of the PCCP themed issue on “Solvation Science”
The emerging topic of “Solvation Science” aims to develop a universal concept of solvation which not only describes solvents in general, but is additionally able to predict the properties of new solvent systems.
Phys. Chem. Chem. Phys., 2015,17, 8295-8296
https://doi.org/10.1039/C5CP90022K
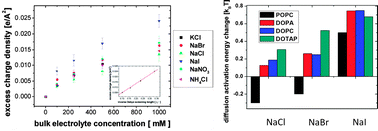
Ion specific effects: decoupling ion–ion and ion–water interactions
Ion-specific effects in aqueous solution, known as the Hofmeister effect, are prevalent in diverse systems. The objective of this paper is to explicitly demonstrate how complex ion–ion and ion–water interactions manifest themselves in the Hofmeister effect based on a series of recent experimental observations.
Phys. Chem. Chem. Phys., 2015,17, 8306-8322
https://doi.org/10.1039/C4CP05992A
Water structure and chaotropicity: their uses, abuses and biological implications
The concept of “water structure” has been invoked to explain all manner of aqueous phenomena.
Phys. Chem. Chem. Phys., 2015,17, 8297-8305
https://doi.org/10.1039/C4CP04564E
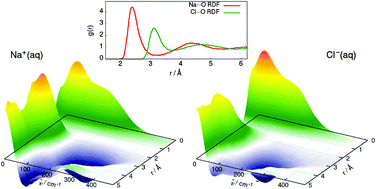
Solvation shell resolved THz spectra of simple aqua ions – distinct distance- and frequency-dependent contributions of solvation shells
Spatial decomposition schemes for infrared spectra reveal the importance of both dipolar couplings and correlations in particle motion in aqueous solutions of Na+ and Cl−.
Phys. Chem. Chem. Phys., 2015,17, 8323-8329
https://doi.org/10.1039/C4CP05268D
A THz/FTIR fingerprint of the solvated proton: evidence for Eigen structure and Zundel dynamics
Zundel (orange), Eigen (red) and hydration water (light blue) contributions to the THz/FIR extinction of the solvated proton.
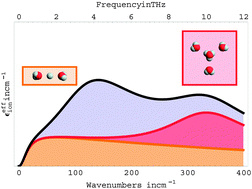
Phys. Chem. Chem. Phys., 2015,17, 11898-11907
https://doi.org/10.1039/C5CP01035G
On the role of gold nanoparticles in the selective photooxidation of 2-propanol over Au/TiO2
Partial insight into the reaction mechanism of the photooxidation of 2-propanol over Au/TiO2. 2-Propanol is activated by hole capture and converted to acetone requiring the presence of O2 as an electron acceptor. The deposited Au nanoparticles are assumed to facilitate the electron transfer from the TiO2 conduction band to adsorbed O2.
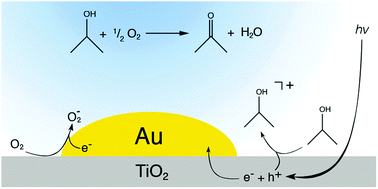
Phys. Chem. Chem. Phys., 2015,17, 10391-10397
https://doi.org/10.1039/C4CP05423G
The intermolecular NOE is strongly influenced by dynamics
New fundamental insights in NOE theory concerning the significance of dynamics and range are presented, using coarse-grained MD simulations.
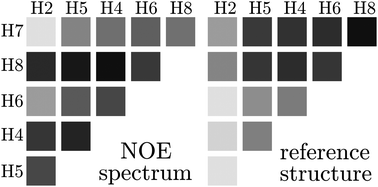
Phys. Chem. Chem. Phys., 2015,17, 8509-8517
https://doi.org/10.1039/C4CP04779F
Understanding the ionic liquid [NC4111][NTf2] from individual building blocks: an IR-spectroscopic study
This study uses complementary spectroscopic methods in combination with quantum chemical calculations to explore at a molecular level the ionic liquid [NC4111][NTf2] from single ions to the bulk.
![Graphical abstract: Understanding the ionic liquid [NC4111][NTf2] from individual building blocks: an IR-spectroscopic study](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5CP00116A&imageInfo.ImageIdentifier.Year=2015)
Phys. Chem. Chem. Phys., 2015,17, 8518-8529
https://doi.org/10.1039/C5CP00116A
On the urea induced hydrophobic collapse of a water soluble polymer
Polymer collapse despite cosolvent binding: solvation of extended coil conformations is entropically penalized, therefore stabilizing compact globular conformations in the coil-globule equilibrium of poly(N-isopropylacrylamide) in aqueous urea solution.
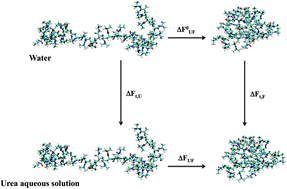
Phys. Chem. Chem. Phys., 2015,17, 8491-8498
https://doi.org/10.1039/C4CP05314A
Exploring volume, compressibility and hydration changes of folded proteins upon compression
We analyze the temperature and pressure dependence of the apparent volume of a protein and its geometrical, interfacial and hydrational contributions.
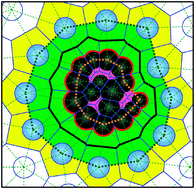
Phys. Chem. Chem. Phys., 2015,17, 8499-8508
https://doi.org/10.1039/C5CP00251F
Monitoring ultrafast intramolecular proton transfer processes in an unsymmetric β-diketone
Electronic excitation of a UV-absorbing unsymmetric β-diketone discloses intramolecular proton transfer among electronic ground as well as excited states.
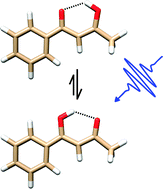
Phys. Chem. Chem. Phys., 2015,17, 8459-8466
https://doi.org/10.1039/C4CP05811A
Solvent effects of 1-ethyl-3-methylimidazolium acetate: solvation and dynamic behavior of polar and apolar solutes
We study the solvation properties of the ionic liquid 1-ethyl-3-methylimidazolium acetate ([eMIM]+[ACE]−) and the resulting dynamic behavior for differently charged model solutes at room temperature via atomistic molecular dynamics (MD) simulations of 500 ns length.

Phys. Chem. Chem. Phys., 2015,17, 8480-8490
https://doi.org/10.1039/C4CP05312E
Structural properties of methanol–water binary mixtures within the quantum cluster equilibrium model
The Quantum Cluster Equilibrium (QCE) method computes cluster distributions and thermodynamic properties of binary methanol–water mixtures in agreement with experiments.
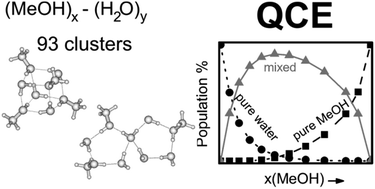
Phys. Chem. Chem. Phys., 2015,17, 8467-8479
https://doi.org/10.1039/C4CP05836D
A femtosecond mid-infrared study of the dynamics of water in aqueous sugar solutions
The influence of sugars on the dynamics of water is relatively long-ranged and involves collective structural effects.
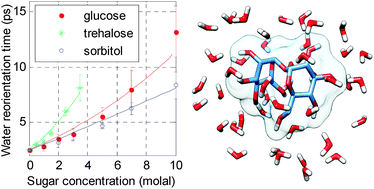
Phys. Chem. Chem. Phys., 2015,17, 8449-8458
https://doi.org/10.1039/C4CP05431H
Ultrafast electron dynamics at water covered alkali adatoms adsorbed on Cu(111)
Here we report on the ultrafast electron dynamics of the alkalis Na, K, and Cs coadsorbed with D2O on Cu(111) surfaces, which we investigated with femtosecond time-resolved two-photon photoemission.
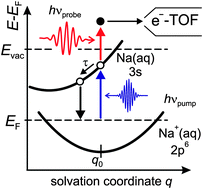
Phys. Chem. Chem. Phys., 2015,17, 8441-8448
https://doi.org/10.1039/C4CP05356G
Hydrogen bonding in a mixture of protic ionic liquids: a molecular dynamics simulation study
We report results of molecular dynamics (MD) simulations characterising the hydrogen bonding in mixtures of two different protic ionic liquids sharing the same cation: triethylammonium-methylsulfonate (TEAMS) and triethylammonium-triflate (TEATF).
Phys. Chem. Chem. Phys., 2015,17, 8431-8440
https://doi.org/10.1039/C4CP05432F
Long-range ion–water and ion–ion interactions in aqueous solutions
Using small-angle X-ray scattering (SAXS), we obtained direct experimental evidence on the structure of hydrated polyatomic anions, with hydration effects starkly different from those of cations (J. Chem. Phys., 2011, 134, 064513).
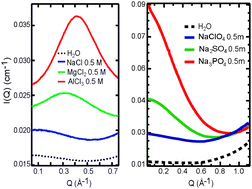
Phys. Chem. Chem. Phys., 2015,17, 8427-8430
https://doi.org/10.1039/C4CP04759A
Determination of partial molar volumes from free energy perturbation theory
Free Energy Perturbation calculations are employed to determine free energies of solvation (ΔGsolv) for benzene and benzene-derivatives at elevated pressures. Absolute and relative partial molar volumes are determined as the pressure derivative of ΔGsolv.
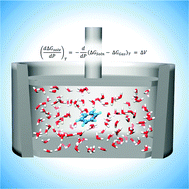
Phys. Chem. Chem. Phys., 2015,17, 8407-8415
https://doi.org/10.1039/C4CP05304D
Evaluation of water displacement energetics in protein binding sites with grid cell theory
The grid cell theory method was used to elucidate perturbations in water network energetics in a range of protein–ligand complexes.
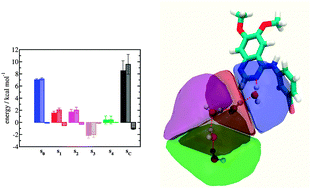
Phys. Chem. Chem. Phys., 2015,17, 8416-8426
https://doi.org/10.1039/C4CP05572A
Systematic evaluation of bundled SPC water for biomolecular simulations
How accurate is bundled SPC water as inner shell solvent for hybrid all-atom/coarse-grained simulations?
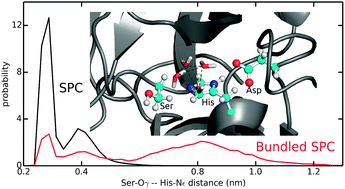
Phys. Chem. Chem. Phys., 2015,17, 8393-8406
https://doi.org/10.1039/C4CP04784B
Photoresponsive ionic liquid crystals based on azobenzene guanidinium salts
The mesomorphic properties and the kinetics of the E/Z-photoisomerization in an ILC matrix of new azobenzene ILCs were investigated.
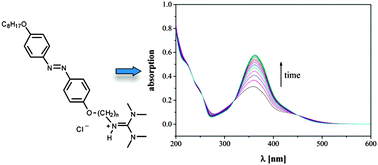
Phys. Chem. Chem. Phys., 2015,17, 8382-8392
https://doi.org/10.1039/C4CP04783D
Prediction of enhanced solvent-induced enantioselectivity for a ring opening with a bifurcating reaction path
A fully atomistic molecular dynamics simulation predicts enhance induction of enantiomeric excess in the products of a reaction with a bifurcating reaction coordinate, when run in a chiral solvent.
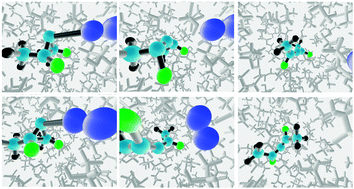
Phys. Chem. Chem. Phys., 2015,17, 8372-8381
https://doi.org/10.1039/C4CP05078A
Representing the potential-energy surface of protonated water clusters by high-dimensional neural network potentials
We report a reactive neural network potential for protonated water clusters that accurately represents the density-functional theory potential-energy surface.
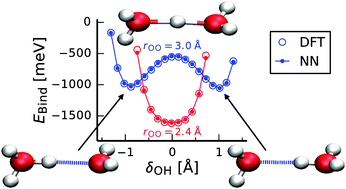
Phys. Chem. Chem. Phys., 2015,17, 8356-8371
https://doi.org/10.1039/C4CP04751F
Modulation of human IAPP fibrillation: cosolutes, crowders and chaperones
The mechanism of human IAPP aggregation is studied in the presence of three different classes of chaperones and crowding agents.
Phys. Chem. Chem. Phys., 2015,17, 8338-8348
https://doi.org/10.1039/C4CP04682J
Cosolvent and crowding effects on the polymerization kinetics of actin
Effects of cosolvents and macromolecular crowding agents on the G-to-F-transformation of actin are studied. Drastic and diverse changes in the lag phase and association rates of polymerizing actin are observed under different solvent conditions.
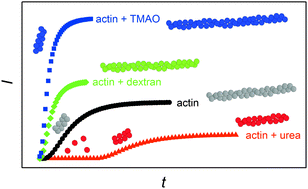
Phys. Chem. Chem. Phys., 2015,17, 8330-8337
https://doi.org/10.1039/C4CP04431B
Non-covalent interactions in water electrolysis: influence on the activity of Pt(111) and iridium oxide catalysts in acidic media
Electrolyte components, which are typically not considered to be directly involved in catalytic processes at solid–liquid electrified interfaces, often demonstrate a significant or even drastic influence on the activity, stability and selectivity of electrocatalysts.
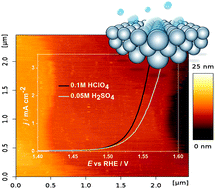
Phys. Chem. Chem. Phys., 2015,17, 8349-8355
https://doi.org/10.1039/C4CP04791E
Long-range influence of steps on water adsorption on clean and D-covered Pt surfaces
Water wets the D-covered Pt(111) surface (right), while it clusters at steps of D-covered Pt(533), (755), and (977) (left).
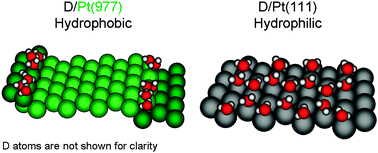
Phys. Chem. Chem. Phys., 2015,17, 8530-8537
https://doi.org/10.1039/C4CP03165B
About this collection
This is the official themed issue of the international Bunsentagung 2015 on the same theme organised by the Deutsche Bunsen-Gesellschaft (DBG) during 14-16 May 2015 at the Ruhr-Universität Bochum in Germany. The issue features high quality and original research on the theme “Solvation Science”, and was Guest Edited by Karina Morgenstern, Dominik Marx, Martina Havenith, and Martin Muhler.