Themed collection Non-Innocent Ligands

Non-innocent ligands
Guest editors Louise Berben, Bas de Bruin and Alan Heyduk introduce this web collection focusing on recent achievements within the field of non-innocent ligands.

Chem. Commun., 2015,51, 1553-1554
https://doi.org/10.1039/C4CC90480J
Metal–ligand bifunctional reactivity and catalysis of protic N-heterocyclic carbene and pyrazole complexes featuring β-NH units
The metal–ligand bifunctional cooperation of protic N-heterocyclic carbene and pyrazole complexes bearing an NH unit at the position β to the metal is surveyed.
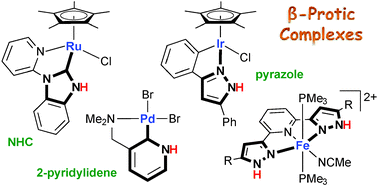
Chem. Commun., 2014,50, 14290-14300
https://doi.org/10.1039/C4CC04457F
Organic azides: “energetic reagents” for the intermolecular amination of C–H bonds
This feature article highlights the potentiality of organic azides (RN3) for the intermolecular amination of sp3 and sp2 C–H bonds. A compendium of employed catalytic systems, together with a discussion of involved mechanisms, is provided.

Chem. Commun., 2014,50, 11440-11453
https://doi.org/10.1039/C4CC03016H
Classical and non-classical redox reactions of Pd(II) complexes containing redox-active ligands
Chemical reactions of a verdazyl radical–palladium complex reveal the verdazyl’s functionality as a redox-active ligand and a “cooperative” (acido-basic) ligand.
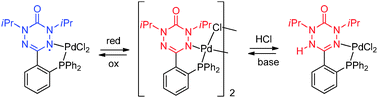
Chem. Commun., 2014,50, 11676-11678
https://doi.org/10.1039/C4CC04863F
Geometric and redox flexibility of pyridine as a redox-active ligand that can reversibly accept one or two electrons
A low-coordinate iron(I) species can reversibly reduce pyridine by one or two electrons, through changes in C–C bonding.
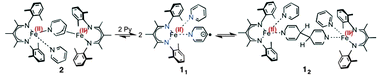
Chem. Commun., 2014,50, 11114-11117
https://doi.org/10.1039/C4CC05495D
Platinum corroles
Platinum has been inserted into corroles for the first time and three oxidized PtIV(corrole˙2−)ArAr′ complexes have been structurally characterized.

Chem. Commun., 2014,50, 11093-11096
https://doi.org/10.1039/C4CC02548B
(Electro)catalytic C–C bond formation reaction with a redox-active cobalt complex
Cooperativity between cobalt and non-innocent ligands in electron transfer processes has been utilized for (electro)catalytic C–C bond formation reactions.

Chem. Commun., 2014,50, 11104-11106
https://doi.org/10.1039/C4CC03309D
An electron transfer series of octahedral chromium complexes containing a redox non-innocent α-diimine ligand
An electron-transfer series of octahedral α-diimine complexes [(HLCy)3Cr]n+(BARF)n (n = 2, 1, 0) has been synthesized and crystallographically characterized.
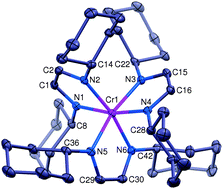
Chem. Commun., 2014,50, 10626-10629
https://doi.org/10.1039/C4CC03332A
Iminosemiquinone radical ligands enable access to a well-defined redox-active CuII–CF3 complex
The reaction of a copper complex bearing iminosemiquinone ligands with a CF3+ source provides an unprecedented CuII–CF3 complex through ligand-based oxidation.
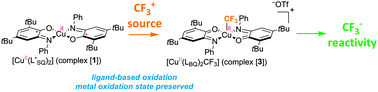
Chem. Commun., 2014,50, 10394-10397
https://doi.org/10.1039/C4CC04487H
Adaptive behavior of a redox-active gallium carbenoid in complexes with molybdenum
A gallium–nitrogen heterocycle derived from 1,2-bis(imino)acenaphthene may serve either as a neutral or an anionic two-electron donor depending on the electronic state of the transition metal.
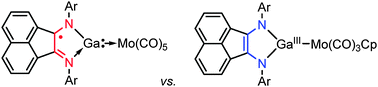
Chem. Commun., 2014,50, 10108-10111
https://doi.org/10.1039/C4CC04019H
Influence of redox non-innocent phenylenediamido ligands on chromium imido hydrogen-atom abstraction reactivity
Reaction of new CpCr[(RN)2C6H4] complexes (R = SiMe3, CH2CMe3, Ph) with organic azides generates chromium imido complexes.
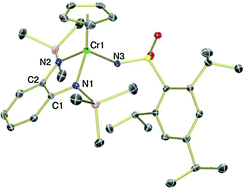
Chem. Commun., 2014,50, 9958-9960
https://doi.org/10.1039/C4CC04545A
Evidence of two-state reactivity in alkane hydroxylation by Lewis-acid bound copper–nitrene complexes
Two state reactivity hypothesis, well established for iron-oxo cores, can also be extended to the copper–nitrenes.
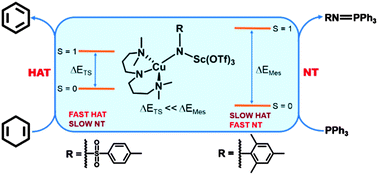
Chem. Commun., 2014,50, 9852-9854
https://doi.org/10.1039/C4CC03754E
Syntheses and electronic structures of μ-nitrido bridged pyridine, diimine iridium complexes
Oxidation states in late transition metal nitrido complexes.
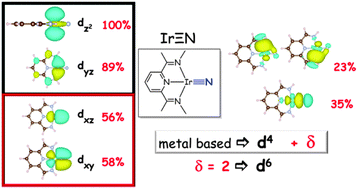
Chem. Commun., 2014,50, 8735-8738
https://doi.org/10.1039/C4CC03624G
Mechanistic investigation of the selective reduction of Meldrum's acids to β-hydroxy acids using SmI2 and H2O
The mechanism of the mono-reduction of Meldrum's acids to β-hydroxy acids employing SmI2–H2O has been elucidated.
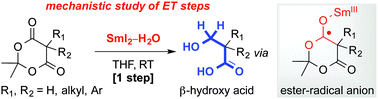
Chem. Commun., 2014,50, 8391-8394
https://doi.org/10.1039/C4CC03216K
Utility of a redox-active pyridine(diimine) chelate in facilitating two electron oxidative addition chemistry at uranium
Tetravalent CpPU(MesPDIMe) uses its [MesPDIMe]3− chelate to perform oxidative addition of halides and dichalcogenides, generating the uranium(IV) family, CpPU(XX′)(MesPDIMe) (X = X′ = I; X = X′ = Cl; X = SePh, X′ = Cl; X = X′ = EPh), which is supported by [MesPDIMe]1− ligand.
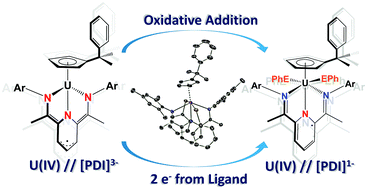
Chem. Commun., 2014,50, 8189-8192
https://doi.org/10.1039/C4CC03355H
Octahedral to trigonal prismatic distortion driven by subjacent orbital π antibonding interactions and modulated by ligand redox noninnocence
Ruthenium and osmium bis(amidodiphenoxides) distort towards trigonal prismatic geometries to minimize aryloxide-to-metal π* interactions, limited by increasing degree of oxidation of the redox-active ligand.
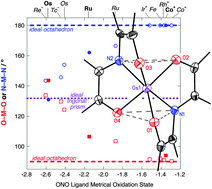
Chem. Commun., 2014,50, 7956-7959
https://doi.org/10.1039/C4CC03404J
Synthesis and ligand-based reduction chemistry of boron difluoride complexes with redox-active formazanate ligands
Mono(formazanate) boron difluoride complexes (LBF2), which show remarkably facile and reversible ligand-based redox-chemistry, were synthesized by transmetallation of bis(formazanate) zinc complexes with boron trifluoride.
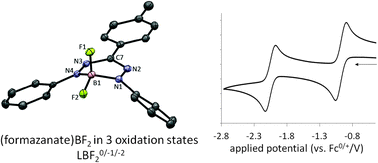
Chem. Commun., 2014,50, 7431-7433
https://doi.org/10.1039/C4CC03244F
About this collection
This ChemComm themed collection publishes contributions from all areas of chemistry that include the use of ligands that are both redox non-innocent and chemically non-innocent. It covers not only synthetic inorganic chemistry and catalysis, but also other applications of non-innocent ligand chemistry. The issue is Guest Edited by Louise Berben, Alan Heyduk and Bas de Bruin. New articles will be added to this collection as they are published.