Mechanistic investigation of the selective reduction of Meldrum's acids to β-hydroxy acids using SmI2 and H2O†
Abstract
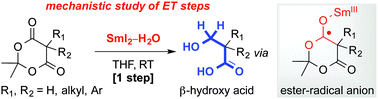
The mechanism of a recently reported first mono-reduction of cyclic 1,3-diesters (Meldrum's acids) to β-hydroxy acids with SmI2–H2O has been studied using a combination of reactivity, deuteration, kinetic isotope and radical clock experiments. Most crucially, the data indicate that the reaction proceeds via reversible electron transfer and that water, as a ligand for SmI2, stabilizes the radical anion intermediate rather than only promoting the first electron transfer as originally proposed.
- This article is part of the themed collection: Non-Innocent Ligands

 Please wait while we load your content...
Please wait while we load your content...