Enantioselective β-alkylation of pyrroles with the formation of an all-carbon quaternary stereocenter†
Abstract
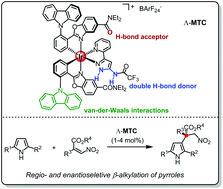
A substitutionally and configurationally inert octahedral chiral-at-metal iridium complex is reported to be an efficient catalyst for the enantioselective Friedel–Crafts alkylation of 2,5-disubstituted pyrroles at the β-position using nitroacrylates as electrophiles. Catalysis is mediated through the ligand sphere of the bis-cyclometalated iridium complex by forming hydrogen bonds and van-der-Waals interactions with the substrates, providing β-alkylated pyrroles regioselectively in 75–98% yield and with 86–99% enantiomeric excess (19 examples). The products are versatile chiral building blocks as demonstrated by the reduction of the nitro group with complete retention of the chiral information.

 Please wait while we load your content...
Please wait while we load your content...