Synthesis of 2,3-dihydrobenzofurans via the palladium catalyzed carboalkoxylation of 2-allylphenols†
Abstract
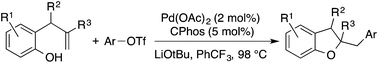
A new Pd-catalyzed alkene carboalkoxylation strategy for the preparation of 2,3-dihydrobenzofurans is described. This method effects the coupling of readily available 2-allylphenol derivatives with aryl triflates to generate a wide range of functionalized 2,3-dihydrobenzofurans in good yields and diastereoselectivities (up to >20 : 1). Use of newly developed reaction conditions that promote anti-heteropalladation of the alkene is essential in order to generate products in high yield.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2016 and Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...