Synthesis of rearranged indole diterpenes of the paxilline type
Abstract
Covering: up to 2021
Rearranged indole diterpenes of the paxilline type comprise a large group of fungal metabolites that possess diverse structural features and potentially useful biological effects. The unique indoloterpenoid motif, which is common to all congeners, was first confirmed by crystallographic studies of paxilline. This family of natural products has fascinated organic chemists for the past four decades and has inspired numerous syntheses and synthetic approaches. The present review highlights efforts that have laid the foundation and introduced new directions to this field of natural product synthesis. The introduction includes a summary of biosynthetic considerations and biological activities, the main body of the manuscript provides a detailed discussion of selected syntheses, and the review concludes with a brief outlook on the future of the field.
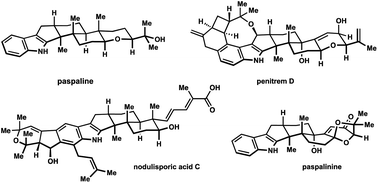
- This article is part of the themed collection: Synthetic strategies for mining the information-rich content of natural products


 Please wait while we load your content...
Please wait while we load your content...