Biosynthesis of cyclopropane in natural products
Abstract
Covering: 2012 to 2021
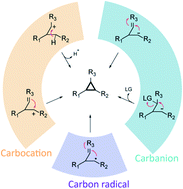
Cyclopropane attracts wide interests in the fields of synthetic and pharmaceutical chemistry, and chemical biology because of its unique structural and chemical properties. This structural motif is widespread in natural products, and is usually essential for biological activities. Nature has evolved diverse strategies to access this structural motif, and increasing knowledge of the enzymes forming cyclopropane (i.e., cyclopropanases) has been revealed over the last two decades. Here, the scientific literature from the last two decades relating to cyclopropane biosynthesis is summarized, and the enzymatic cyclopropanations, according to reaction mechanism, which can be grouped into two major pathways according to whether the reaction involves an exogenous C1 unit from S-adenosylmethionine (SAM) or not, is discussed. The reactions can further be classified based on the key intermediates required prior to cyclopropane formation, which can be carbocations, carbanions, or carbon radicals. Besides the general biosynthetic pathways of the cyclopropane-containing natural products, particular emphasis is placed on the mechanism and engineering of the enzymes required for forming this unique structure motif.


 Please wait while we load your content...
Please wait while we load your content...