Effect of Na+ and K+ on the cucurbituril-mediated hydrolysis of a phenyl acetate†
Abstract
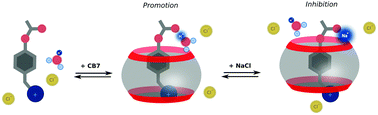
The environment around the active site affects the catalytic activity of enzymes. Studying the cucurbit[7]uril-promoted acid hydrolysis of a cationic phenyl acetate derivative, we found that the hydrophobic cavity of the macrocycle screens the reaction centre from the positively charged neighbouring group. Moreover, the chelation of alkali metal cations with the cucurbit[7]uril portal and acetyl group of the substrate reduces the hydrolysis rate of the encapsulated ester in an aqueous solution. This type of inhibition corresponds to a rare uncompetitive model in contrast to the more common competitive model that relies on substrate displacement.
- This article is part of the themed collection: ChemComm Milestones – First Independent Articles


 Please wait while we load your content...
Please wait while we load your content...