Abstract
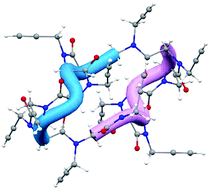
Enantiomorphic right- and left-handed polyproline type I helices in four cyclic dodecapeptoids with methoxyethyl and propargyl side chains are observed for the first time by single crystal X-ray diffraction. The peculiar absence of NH⋯OC hydrogen bonds in peptoids unveils the role of intramolecular backbone-to-backbone CO⋯CO interactions and CH⋯OC hydrogen bonds in the stabilization of the macrocycle conformation. Moreover, intramolecular backbone-side chain C5 CH⋯OC hydrogen bonds emerge as a stabilizing factor.


 Please wait while we load your content...
Please wait while we load your content...