Regio- and stereoselective synthesis of functionalized and fused heterocycles from Morita–Baylis–Hillman adducts of dicyclopentadienone†
Abstract
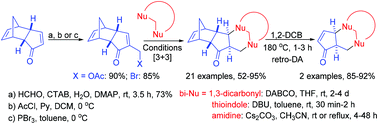
An efficient protocol for the Morita–Baylis–Hillman (MBH) reaction of dicyclopentadienone using CTAB has been developed. The MBH adduct was subsequently transformed to its acetate and bromide derivatives. The 1,3-bielectrophilic character of the MBH acetate and bromide was further explored with various 1,3-binucleophiles to construct various oxa-, thia- and aza-heterocycles. These reactions proceed through a cascade double Michael addition of 1,3-binucleophiles to the MBH acetate/bromide under basic conditions. Amenability of these reactions to scale-up and applications of the products in the synthesis of cyclopentenone-fused chromenones and thiopyranoindoles have been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...