Cu(OTf)2-catalyzed C3 aza-Friedel–Crafts alkylation of indoles with N,O-acetals†
Abstract
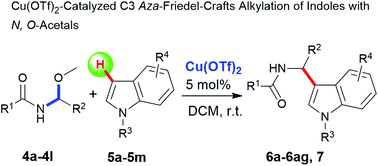
An efficient approach to access functionalized indole derivatives has been developed through Cu(OTf)2-catalyzed C3 aza-Friedel–Crafts alkylation of substituted indoles 5a–5m, N-methyl-pyrrole with linear N,O-acetals 4a–4l. As a result, a series of C3 amide aza-alkylated indole derivatives 6a–6ag and 7 were synthesized in moderate to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...