Nucleophile-induced transformation of phenoxathiin-based thiacalixarenes†
Abstract
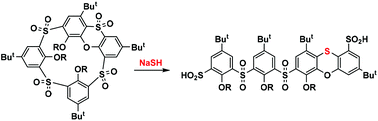
Oxidized phenoxathiin-based macrocycles, easily accessible thiacalix[4]arene derivatives, consist of a unique set of structural elements representing a key prerequisite for the unexpected reactivity described in this paper. As proposed, the internal strain, imposed by the presence of a heterocyclic moiety, together with a number of electron-withdrawing groups (SO2) opens the way to the cleavage of the macrocyclic skeleton through a cascade of three SNAr reactions triggered by the nucleophilic attack of an SH− anion. The whole transformation, which is unparalleled in classical calixarene chemistry, leads to unique linear sulfinic acid derivatives with a rearranged phenoxathiin moiety that can serve as building blocks for macrocyclic systems of a new type.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...