Enhanced duplex- and triplex-forming ability and enzymatic resistance of oligodeoxynucleotides modified by a tricyclic thymine derivative†
Abstract
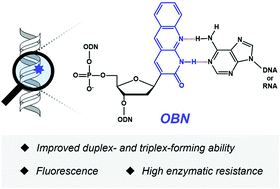
We designed and synthesized an artificial nucleic acid, [3-(1,2-dihydro-2-oxobenzo[b][1,8]naphthyridine)]-2′-deoxy-D-ribofuranose (OBN), with a tricyclic structure in a nucleobase as a thymidine analog. Oligodeoxynucleotides (ODNs) containing consecutive OBN displayed improved duplex-forming ability with complementary single-stranded (ss) RNA and triplex-forming ability with double-stranded DNA in comparison with ODNs composed of natural thymidine. OBN-modified ODNs also displayed enhanced enzymatic resistance compared with ODNs with natural thymidine and phosphorothioate modification, respectively, due to the structural steric hindrance of the nucleobase. The fluorescence spectra of OBN-modified ODNs showed sufficient fluorescence intensity with ssDNA and ssRNA, which is an advantageous feature for fluorescence imaging techniques of nucleic acids with longer emission wavelengths than bicyclic thymine (bT).
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...