Diverse privileged N-polycyclic skeletons accessed from a metal-free cascade cyclization reaction†
Abstract
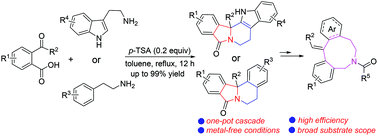
An exquisite metal-free cascade cyclization reaction of 2-acylbenzoic acids with amines was developed, which provided a powerful method for the one-pot synthesis of diverse isoindoloisoquinoline and benzoindolizinoindole derivatives. This protocol avoided the use of metal catalysts, proceeded with high efficiency and had broad substrate scope. These resulting products could be transformed into tertiary amines under the reduction of LiAlH4/AlCl3, followed by the Hofmann elimination offering lots of nitrogen-containing nine-membered ring compounds in excellent yields. All synthesized products containing fused N-polycyclic skeletons were difficult to be constructed using traditional methods and they have a wide range of applications in the pharmaceutical area.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...